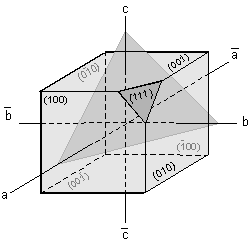
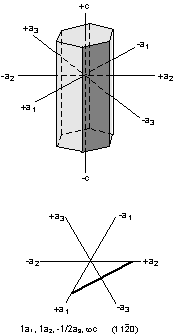
We've now seen how crystallographic axes can be defined for the various
crystal systems. Two important points to remember are that
- The lengths of the crystallographic axes are controlled by the
dimensions of the unit cell upon which the crystal is based.
- The angles between the crystallographic axes are controlled by the
shape of the unit cell.
We also noted last time that the relative lengths of the
crystallographic axes control the angular relationships between crystal
faces. This is true because crystal faces can only develop along
lattice points. The relative lengths of the crystallographic axes
are called axial ratios, our first topic of discussion.
Axial Ratios
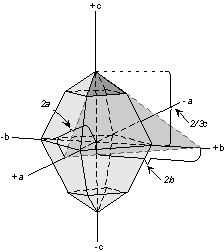
Axial ratios are defined as the relative lengths of the
crystallographic axes. They are normally taken as relative to the
length of the b crystallographic axis. Thus, an axial ratio is
defined as follows:
Axial Ratio = a/b : b/b : c/b
where a is the actual length of the a crystallographic axis, b, is the
actual length of the b crystallographic axis, and c is the actual length
of the c crystallographic axis.
- For Triclinic, Monoclinic, and Orthorhombic crystals, where the
lengths of the three axes are different, this reduces to
a/b : 1 : c/b (this is usually shortened to a : 1 : c)
1 : 1 : c/b (this is usually shorted to 1 : c)
1 : 1 : 1 (this is usually shorted to 1)
1 : 1 : 1: c/a (usually shortened to 1 : c)
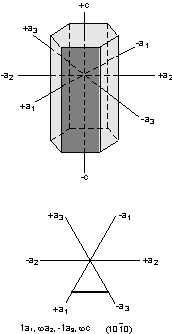
Modern crystallographers can use x-rays to determine the
size of the unit cell, and thus can determine the absolute value of the
crystallographic axes. For example, the mineral quartz is hexagonal,
with the following unit cell dimensions as determined by x-ray
crystallography:
a1 = a2 = a3 = 4.913Å
c = 5.405Å
where Å stands for Angstroms = 10-10 meter.
Thus the axial ratio for quartz is
1 : 1 : 1 : 5.405/4.913
or
1: 1 : 1 : 1.1001
which simply says that the c axis is 1.1001 times longer
than the a axes.
For orthorhombic sulfur the unit cell dimensions as
measured by x-rays are:
a = 10.47Å
b = 12.87Å
c = 24.39Å
Thus, the axial ratio for orthorhombic sulfur is:
10.47/12.87 : 12.87/12.87 : 24.39/12.87
or
0.813 : 1 : 1.903
Because crystal faces develop along lattice points, the angular relationship between faces must depend on the relative lengths
of the axes. Long before x-rays were invented and absolute unit
cell dimensions could be obtained, crystallographers were able to
determine the axial ratios of minerals by determining the angles between
crystal faces. So, for example, in 1896 the axial ratios of
orthorhombic sulfur were determined to be nearly exactly the same as those
reported above from x-ray measurements.
In a later lecture we will see how we can determine axial
ratios from the angular relationships between faces. First, however
we must determine how we can name, or index faces of crystals and define
directions within crystals.
|