Since the Si - O bonds are strong covalent bonds and since the structure is interlocking, the tectosilicate minerals tend to have a high hardness.
| EENS 2110 |
Mineralogy |
| Tulane University |
Prof. Stephen A. Nelson |
|
Tectosilicates, Carbonates, Oxides, & Accessory Minerals |
|
|
|
|
Tectosilicates (Framework Silicates) |
|
The tectosilicates or framework silicates have a structure wherein all of
the 4 oxygens of SiO4-4 tetrahedra are shared with
other tetrahedra. The ratios of Si to O is thus 1:2.
Since the Si - O bonds are strong covalent bonds and since the structure is interlocking, the tectosilicate minerals tend to have a high hardness. |
 |
|
SiO2 Minerals
There are nine known polymorphs of SiO2, one of which does not occur naturally. These are:
|
| Stishovite and Coesite are high pressure forms of SiO2, and thus have much higher densities and refractive indices than the other polymorphs. Stishovite is the only polymorph where the Si occurs in 6 fold (octahedral) coordination with Oxygen, and this occurs due to the high pressure under which the mineral forms. Both Stishovite and Coesite have been found associated with meteorite impact structures. | 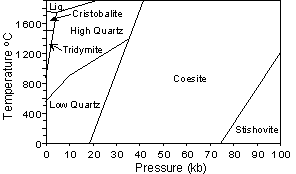 |
|
At low pressure with decreasing temperature, SiO2 polymorphs
change from high Cristobalite - Low Cristobalite - High Tridymite - Low
Tridymite - High Quartz - Low Quartz. The high to low
transformations are all displacive transformations. Since displacive
transformations require little rearrangement of the crystal structure and
no change in energy, the high (β) polymorphs do
not exist at the surface of the earth, as they will invert to the low (α)
polymorphs as temperature is lowered.
Transformations between α Cristobalite, α Tridymite, and α Quartz, however, as
well as between the high pressure polymorphs and Quartz, are
reconstructive transformations. Since reconstructive transformations
require significant structural rearrangement and significant changes in
energy, they occur slowly, and the high temperature and high pressure
polymorphs can occur as metastable minerals at the Earth's surface.
|
Quartz Quartz is hexagonal and commonly occurs as crystals ranging in size form microscopic to crystals weighing several tons. Where it crystallizes unhindered by other crystals, such as in cavities in rock or in a liquid containing few other crystals, it shows well-developed hexagonal prisms and sometimes showing apparent hexagonal pyramids or dipyramid. When it crystallizes in an environment where growth is inhibited by the surroundings, it rarely show crystal faces. It is also found as microcrystalline masses, such as in the rock chert, and as fibrous masses, such as in chalcedony.
As visible crystals, Quartz is one of the more common rock forming minerals. It occurs in siliceous igneous rocks such as volcanic rhyolite and plutonic granitic rocks. It is common in metamorphic rocks at all grades of metamorphism, and is the chief constituent of sand. Because it is highly resistant to chemical weathering, it is found in a wide variety of sedimentary rocks. Several varieties of Quartz can be found, but these are usually only distinguishable in hand specimen. Rock Crystal - clear Quartz in distinct crystals - usually found growing in open cavities in rock. Amethyst - violet colored Quartz, with the color resulting from trace amounts of Fe in the crystal. Rose Quartz - a pink colored variety, that usually does not show crystal faces, the color resulting from trace amounts of Ti+4. Smokey Quartz - a dark colored variety that may be almost black, usually forming well-formed crystals. The color appears to result from trace amounts of Al+3 in the structure. Citrine - a yellow colored variety. Milky Quartz - a white colored variety with the color being due to fluid inclusions. Milky Quartz is common in hydrothermal veins and pegmatites.
A fibrous variety of Quartz is called Chalcedony. It is usually brown to gray to translucent with a waxy luster. It is found lining or filling cavities in rock where it was apparently precipitated from an aqueous solution. When it shows bands of color, it is commonly called by the following names: Carnelian - red colored Chalcedony Chrysoprase - apple-green colored as a result of coloration from NiO. Agate - alternating curving layers of Chalcedony with different colors or different porosities. Onyx - alternating layers of Chalcedony of different colors or porosities arranged in parallel planes. Bloodstone - green Chalcedony containing red spots of jasper (see below).
Very fined grained aggregates of cryptocrystalline quartz makes up rock like Flint and Chert. Flint occurs as nodules in limestone, whereas chert is a layered rock deposited on the ocean floor. The red variety of flint is called Jasper, where the color results from inclusions of hematite. Optical Properties Quartz is uniaxial positive with a low relief and low birefringence, thus exhibited only 1o gray to 1o white interference colors. In thin section it is almost always colorless when viewed without the analyzer inserted. One of its most distinguishing properties in thin section is that it usually has a smooth, almost polished-like surface texture. Quartz is easily distinguished from the Feldspars by the biaxial nature of feldspars, and from Nepheline which is uniaxial negative. Apatite, has similar birefringence to quartz, but is uniaxial negative and has a very high relief. In Chalcedony, the fibers are usually elongated perpendicular to the c-crystallographic axis and thus are length fast. Normal quartz, when it show an elongated habit, is elongated parallel to the c axis, and is thus length slow.
|
| Tridymite
Tridymite is the high temperature polymorph of SiO2. Thus, it is only commonly found in igneous rocks that have been cooled rapidly to surface temperatures, preventing the slow transformation to quartz, the stable form of SiO2 at surface temperatures. Because of this, we only expect to find Tridymite in siliceous volcanic rocks like rhyolites, where it commonly occurs as wedge shaped crystals in cavities in the rock. In volcanic rocks, Tridymite is commonly associated with Cristobalite and Sanidine. |
|
Optical Properties Tridymite usually occurs as orthorhombic or monoclinic wedge shaped crystals with a positive 2V between 40 and 90o. The wedge shape of the crystals is the result of twinning on {110}, and usually as 2 to 3 twinned individuals. Although it has similar birefringence to quartz and feldspar, it has lower refractive indices, and thus shows negative relief compared to quartz and feldspars. Cristobalite is also a high temperature SiO2 polymorph, and thus has a similar occurrence to Tridymite. It also occurs in thermally metamorphosed sandstones. In volcanic rocks it can occur both as a lining in open cavities, and as fine grained crystals in the groundmass of the rock. Optical Properties Cristobalite is tetragonal and thus uniaxial. It has a negative optic sign and shows lower relief than quartz, but has similar birefringence. Opal Opal is amorphous, and thus a mineraloid, with a formula - SiO2.nH2O. |
| Feldspars
The feldspars are the most common minerals in the Earth's crust. They consist of three end-members: KAlSi3O8 - Orthoclase (or), NaAlSi3O8 - Albite (ab), and CaAl2Si2O8 -Anorthite (an) KAlSi3O8 and NaAlSi3O8 form a complete solid solution series, known as the alkali feldspars and NaAlSi3O8 and CaAl2Si2O8 form a complete solid solution series known as the plagioclase feldspars. The feldspars have a framework structure, consisting of SiO4 tetrahedra sharing all of the corner oxygens. However, in the alkali feldspars 1/4 of the Si+4 ions are replaced by Al+3 and in the plagioclase feldspars 1/4 to 1/2 of the Si+4 ions are replaced by Al+3. This allows for the cations K+, Na+, and Ca+2 to be substituted into void spaces to maintain charge balance. |
| Compositions of natural feldspars are shown in the diagram below based on the 3 components - NaAlSi3O8, - Albite (ab), KAlSi3O8 - Orthoclase (or) and CaAl2Si2O8. The Alkali Feldspars form a complete solid solution between ab and or, with up to 5% of the an component. The high temperature more K-rich variety is called Sanidine and the more Na-rich variety is called anorthoclase. |
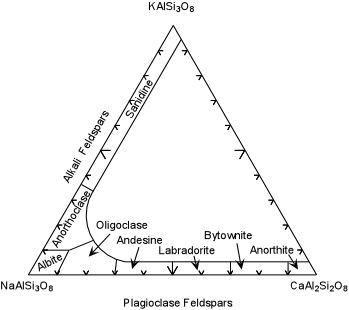 |
| The plagioclase feldspars are a complete solid solution series between
ab and an, and can contain small amounts of the or component. Names
are given to the various ranges of composition, as shown here in the
diagram are:
|
| Plagioclase Feldspars
Plagioclase is the most common feldspar. It forms initially by crystallization from magma. The plagioclase solid solution series is coupled solid solution where the substitution is: Na+1Si+4 <=> Ca+2Al+3 Thus, the general chemical formula for plagioclase can be written as: CaxNa1-xAl1+xSi3-xO8 where x is between 0 and 1.
|
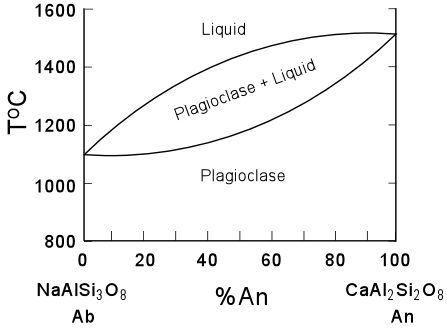
| The phase diagram for the plagiocalse series is shown here, and shows that the Anorthite component has a higher melting temperature the than the Albite component. Thus, on crystallization, higher temperatures will favor more An-rich plagioclase which will react with the liquid to produce more Ab-rich plagioclase on cooling. |
 |
|
Plagioclase occurs in basalts, andesites, dacites, rhyolites, gabbros, diorites, granodiorites, and granites. In most of these igneous rocks, it always shows the characteristic albite twinning. Plagioclase also occurs in a wide variety of metamorphic rocks, where it is usually not twinned. In such rocks where the plagioclase is not twinned, it is difficult to distinguish from the alkali feldspars. Plagioclase can be a component of clastic sedimentary rocks, although it is less stable near the Earth's surface than alkali feldspar and quartz, and usually breaks down to clay minerals during weathering. |
|
Properties In hand specimen, plagioclase is most commonly white colored and shows perfect {100} and good {010} cleavage. It is most easily identified and distinguished from quartz, sanidine, orthoclase, and microcline, by its common polysynthetic twinning on {010}. If this twinning is not present, plagioclase can still be distinguished from quartz by its cleavage, but cannot easily be distinguished from the alkali feldspars. If both plagioclase and alkali feldspar occur in the same rock, the two can usually be distinguished by differences in color or differences in the extent of weathering. |
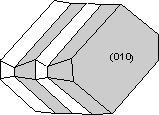 |
| In thin section, plagioclase commonly shows the characteristic albite polysynthetic twinning. This twinning is the most characteristic identifying feature of plagioclase, and makes its identification easy when present. Although some cross-hatched twinning may also occur in plagioclase, it is always very simple with only one or two cross twins per grain. Thus, be careful not to identify plagioclase as microcline. The cross-hatched twinning in microcline is always much more complex. | 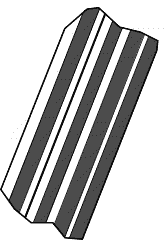 |
| Plagioclase often shows zoning.
This is exhibited by the extinction position changing from the rim to the
core of the crystal. Remember that zoning is caused by incomplete reaction
of crystals with liquid during cooling of a solid solution. Often the
zoning is very complex, and is sometimes oscillatory. Normal zoning would
show Ca - rich cores and Na - rich rims, but reverse zoning is possible
under certain conditions.
In metamorphic rocks plagioclase may not show twinning making it difficult to distinguish from orthoclase. The two can be distinguished by staining the thin section with stains that make the K-feldspars one color and the more Ca-rich feldspars another color. In this class, we will not have time to look at these staining techniques. You should, however, be aware, that such staining techniques exist, so that if you need them in the future, you can use them. The optical properties of the plagioclase series vary widely as a
function of composition of the plagioclase. In general, all plagioclases
show low order interference colors, and thus, low birefringence. Optic
sign and 2V vary widely, and are thus, not very distinguishing features of
plagioclase. Although, as you have seen in lab, it is possible to estimate
the composition of plagioclase from a combination of extinction angle and twinning. |
Alkali Feldspars (K,Na)AlSi3O8 As an alkali feldspar cools from high temperature to lower temperature, the crystal structure changes from that of sanidine, which is monoclinic, through orthoclase, also monoclinic, but with a different crystal structure than sanidine, to microcline, which is triclinic. These transformations are order-disorder transformations, and thus require large amounts of time. Furthermore, if the feldspar is allowed to cool very slowly, then exsolution will occur, and the solid solution will separate into a Na-rich phase and a K-rich phase. Thus, one expects to find sanidine in rocks that were cooled very rapidly from high temperature, i.e. volcanic rocks. Orthoclase and microcline will be found in plutonic igneous rocks (cooled slowly at depth in the earth) and in metamorphic rocks. In addition, in the plutonic rock types if the cooling takes place slowly enough, then perthitic exsolution lamellae may also form. All of the alkali feldspars have low relief and low birefringence. Thus
the interference colors may range up to 1o white. Since this is the same
interference color we expect for quartz, care must be taken to avoid
confusing feldspars and quartz. Sanidine Sanidine generally occurs with an equant habit (almost square) and shows perfect {001} and {010} cleavages, which readily distinguish it from quartz. Rarely does sanidine show twinning, but when it does, it is usually simple twinning. Optic axis figures will only be found on sections showing both cleavages. Sanidine is optically negative with a 2V of 20 - 50o. This distinguishes it from quartz, which is uniaxial positive, and from the other alkali feldspars which show larger values of 2V. Orthoclase Orthoclase is a common alkali feldspar in granitic rocks and K - Al rich metamorphic rocks. It often shows perfect {001} and {010} cleavages which will distinguish it from quartz. Also, quartz usually shows a smooth surface texture, while orthoclase appears much rougher. Orthoclase is also biaxial, which further distinguishes it from quartz. The 2V of orthoclase varies from 60 to 105o, and thus it may be either positive or negative. The 2V angle distinguishes orthoclase from sanidine, but is otherwise not very useful because of the its wide range. Microcline Microcline is the lowest temperature form of alkali feldspar. Upon cooling, orthoclase must rearrange its structure from monoclinic to triclinic. When this happens, twinning usually results. The twinning characteristic of microcline is a combination of albite twinning and pericline twinning. This results in a cross-hatched pattern (often called tartan twinning) that is the most distinguishing characteristic of microcline. Anorthoclase Anorthoclase is a Na - rich feldspar with approximately equal amounts of the Anorthite (Ca) and orthoclase (K) components. Generally anorthoclase occurs in Na - rich volcanic rocks. Like the other alkali feldspars, it has perfect {001} and {010} cleavages. Sections showing both of the cleavages are best for determining the optic sign and 2V. Anorthoclase sometimes shows twinning, but generally not the multiple twinning seen in the plagioclase feldspars, but a cross-hatched twinning similar to that seen in microcline, but on a very fine scale. Anorthoclase, like sanidine shows a low 2V of 5 to 20o, and is optically negative. Anorthoclase can sometimes be distinguished from sanidine by the fact that anorthoclase usually forms crystals with a tabular, elongated habit, while sanidine forms crystals with a more equant habit. |
| Feldspathoids
The feldspathoid group of minerals are SiO2 poor, alkali rich minerals that occur in low SiO2, high Na2O - K2O igneous rocks. In general, these minerals are not compatible with quartz, and therefore, are rarely, if ever, seen in rocks that contain quartz. They do, however, often occur with feldspars. Because of the alkalic nature of the rocks that contain feldspathoids, associated pyroxenes and amphiboles are of the sodic variety, i.e. aegerine or riebeckite. The main feldspathoids are Nepheline (Na,K)AlSiO4, Kalsilite KAlSi2O6, and Leucite KAlSi2O6. At high temperature there is complete solid solution between Nepheline and Kalsilite, but at low temperature Nepheline can contain only about 12 wt% K2O. Other similar members of the feldspathoid group are:
Nepheline Nepheline occurs in both volcanic and plutonic alkaline igneous rocks. In hand specimen, Nepheline is difficult to distinguish from the feldspars, and thus must usually be identified by its association with other alkalic minerals. Nepheline has a yellowish colored alteration product, called cancrinite. Nepheline is hexagonal, and thus uniaxial, making it easy to distinguish from the feldspars. Furthermore, it is optically negative, making it distinguishable from quartz. It usually shows no cleavage, has low birefringence, and low relief (refractive indices are smaller than the feldspars). The only other common mineral with which nepheline could be confused is apatite, which is also uniaxial negative. Apatite, however, shows much higher relief than does nepheline. Sodalite Leucite
|
|
Oxides The oxide minerals are very common and usually occur as accessory minerals in all kinds of rocks. The most common oxide minerals are the following: Corundum - Al2O3 Spinel
- MgAl2O4 Chromite - Fe+2Cr2O4 Magnetite - Fe3O4 Ilmenite
- FeTiO3
Hematite - Fe2O3 Hematite is one of the most important ores of Fe. It is more oxidized than Magnetite, and thus forms as an alteration product of magnetite as well as other Fe bearing minerals. In most unaltered igneous rocks, hematite occurs as a component of Ilmenite in solid solution. Hematite is hexagonal, but rarely occurs in crystals where its symmetry can be determined. It is found in a variety of forms, ranging from oolitic spherules, to massive fine grained aggregates, to botryoidal masses. It is most easily distinguished by its black to dark red color and reddish brown streak. In thin section it is not easily distinguished from other opaque oxide minerals. |
| Iron-Titanium Oxide Geothermometer
Under magmatic conditions, Ilmenite and Hematite form a complete solid solution series, often called the rhombohedral series since both minerals crystallize in the hexagonal system. Similarly Magnetite and Ulvospinel form a complete solid solution series, called the spinel series. |
| The possible ranges of solid solution are shown in the diagram to the right. Coexisting compositions (as illustrated by the tie line) depend on temperature and the fugacity (similar to partial pressure) of Oxygen. If magma is rapidly cooled so as to preserve the compositions of the high temperature solid solutions, it is possible to calculate the temperature and fugacity of Oxygen that were present just before eruption of the magma. Minerals that allow for determination of the temperature of formation of minerals are referred to as a geothermometer. The example illustrated here is an important one, called the Iron-Titanium Oxide Geothermometer. | 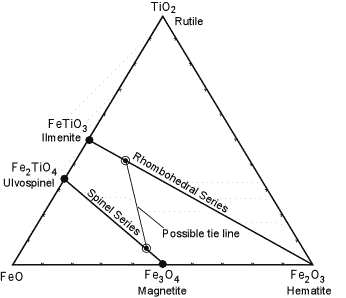 |
|
Carbonates The carbonates are an important group of minerals near the Earth's surface. Carbonate minerals make up the bulk of limestones and dolostones. Are found as cementing agents in clastic sedimentary rocks, and make up the shells of many organisms. The carbonates are based on the CO3-2 structural unit, which has carbon surrounded by 3 oxygens in triangular coordination. Thus each Oxygen has a residual charge of -2/3. In the carbonate structure, no two triangles share the corner oxygens and the C-O bonds are highly covalent. There are three structural types of carbonates: |
| Calcite Group | Aragonite Group | Dolomite Group |
| Calcite CaCO3 | Aragonite CaCO3 | Dolomite CaMg(CO3)2 |
| Magnesite MgCO3 | Witherite BaCO3 | Ankerite CaFe(CO3)2 |
| Siderite FeCO3 | Strontianite SrCO3 | |
| Rhodochrosite MnCO3 | Cerussite PbCO3 | |
| Smithsonite ZnCO3 |
| In addition, there are the hydroxyl Cu carbonates - Malachite, Cu2CO3(OH)2
and Azurite Cu3(CO3)2(OH)2.
The Calcite Group The calcite group minerals are all hexagonal. They have Ca, Mg,
Fe, Mn, or Zn divalent cations in 6-fold coordination with the CO3-2
groups, in a structure that is similar to that of NaCl. All members
of this group show rhombohedral cleavage {01 Calcite CaCO3- The most common carbonate mineral is calcite. It is the principal constituent of limestone and its metamorphic equivalent - marble. Deposits of fine grained calcite in powder form are referred to as chalk. It forms the cementing agent in many sandstones, and is one of the more common minerals precipitated by living organisms to form their skeletal structures. Calcite is also precipitated from groundwater where it form veins, or in open cavities like caves and caverns can form the cave decorations - like stalactites and stalagmites, and encrustations. It is also precipitated from hot springs where it is called travertine. Calcite does occur in rare igneous rocks called carbonatites. These form from carbonate magmas. Calcite is also precipitated from hydrothermal fluids to form veins associated with sulfide bearing ores. Properties Magnesite MgCO3 Siderite FeCO3 Rhodochrosite
MnCO3 |
The Aragonite Group The Aragonite group of minerals are all orthorhombic, and can thus be distinguished from minerals of the calcite group by their lack of rhombohedral cleavage. Aragonite (CaCO3) is the most common mineral in this group. |
| Aragonite is the higher pressure form of CaCO3 but, nevertheless occurs and forms at surface temperatures and pressures. When found in metamorphic rocks it is a good indicator of the low temperature, high pressure conditions of metamorphism, and is thus commonly found in Blueschist Facies metamorphic rocks along with Glaucophane. Water containing high concentrations of Ca and carbonate can precipitate Aragonite. Warm water favors Aragonite, while cold water favors calcite, thus Aragonite is commonly found as a deposit of hot springs. Aragonite can also form by biological precipitation, and the pearly shells of many organisms are composed of Aragonite. Fine needle-like crystals of Aragonite are produced by carbonate secreting algae. | 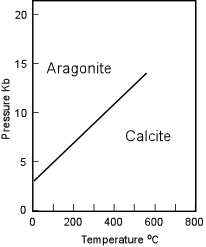 |
Properties |
| The Dolomite Group Dolomite - CaMg(CO3)2 and Ankerite - CaFe(CO3)2 form a complete solid solution series, although because Mg-rich environments are much more common than Fe-rich environments, Mg-rich dolomites are much more common than Ankerites. Ankerite is common mineral in Pre-Cambrian iron formations. Dolomite is a common constituent of older limestones, probably the result of secondary replacement of original calcite. It is also found as dolomitic marbles, and in hydrothermal veins.. |
| Dolomite is a unique chemical composition, as can be seen in the Magnesite - Calcite phase diagram shown here. Two solvi exist at low temperatures. Thus, any high Mg-calcite - dolomite solid solutions that might exist at high temperatures would form nearly pure calcite and pure dolomite at surface temperatures, and similarly, any Magnesite - Dolomite solid solutions that might exist at high temperatures would form nearly pure Magnesite and pure Dolomite at low temperatures. Thus, Magnesite and Dolomite commonly occur together, as do Calcite and Dolomite. | 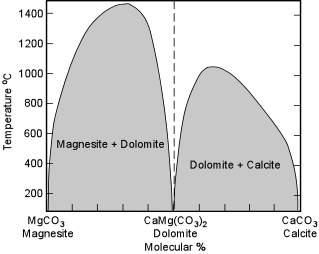 |
| PropertiesDolomite, and therefore rocks containing large amounts of dolomite, like dolostones, is
easily distinguished by the fact that dolomite only fizzes in cold dilute HCl if broken down to a
fine powder. Also, dolostones tend to weather to a brownish color rock, whereas
limestones tend to weather to a white or gray colored rock. The brown color of
dolostones is due to the fact that Fe occurs in small amounts replacing some of the Mg in
dolomite.
In thin section it is more difficult to distinguish from calcite, unless it is twined. In order to facilitate its identification in thin section, the sections are often stained with alizarin red S. This turns calcite pink, but leaves the dolomite unstained. |
| If calcite and dolomite are twinned, they are easily distinguishable from one another. Calcite shows twin lamellae that are parallel to the rhombohedral cleavage traces and parallel to the long direction of the cleavage rhombs. | 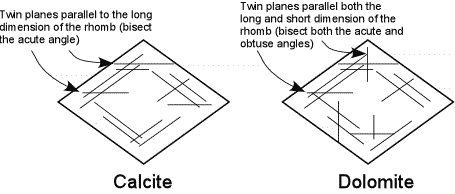 |
| Thus, the lamellae bisect the acute angle between the cleavages. Dolomite also has twins parallel to the cleavage faces and parallel to the long direction of the rhombs, but also has twin lamellae that are parallel to the short dimension of the rhomb. Thus, dolomite would also show twin lamellae that would bisect the obtuse angle between the cleavage traces. |
Apatite Ca5(PO4)3(OH,F) Apatite is another very common and almost ubiquitous (always present) accessory mineral in igneous rocks and many metamorphic rocks. If the rock contains any phosphorous it is usually found in apatite. Apatite is hexagonal, hence uniaxial with a negative optic sign. Its refractive indices ω = 1.624 to 1.666 and ε = 1.629 to 1.667 are higher than both quartz and nepheline, giving apatite a higher relief than these minerals. Its birefringence, expressed as 1o gray interference colors is similar to that of quartz and nepheline. Quartz, however, is optically positive. Nepheline, while optically negative, shows much lower relief than does apatite. The crystal form of apatite is usually distinctive. If cut parallel to {0001}, it usually has a hexagonal outline. If cut parallel to the C axis, it appears as doubly terminated prisms. |
Examples of questions on this material that could be asked on an exam
|