W0-1X2Y5Z8022(OH,F)2
where W = Na+1 or K+1 in the A site with 10 to 12 fold coordination.
X = Ca+2, Na+1, Mn+2, Fe+2, Mg+2, Fe+3, in an M4 site with 6 to 8 fold coordination.
| EENS 212 |
Petrology |
| Prof. Stephen A. Nelson |
Tulane University |
|
Amphiboles & Phyllosilicates |
|
|
|
|
Inosilicates (Double Chain Silicates) - The Amphiboles |
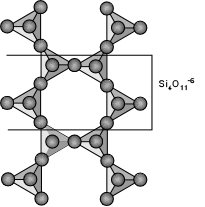
| The amphibole group of minerals is based on the double-chain silicate
structure as shown here. The basic structural unit is (Si4O11)-6.
The structural formula can be written as:
W0-1X2Y5Z8022(OH,F)2 where W = Na+1 or K+1 in the A site with 10 to 12 fold coordination. X = Ca+2, Na+1, Mn+2, Fe+2, Mg+2, Fe+3, in an M4 site with 6 to 8 fold coordination. |
 |
| Y = Mn+2, Fe+2, Mg+2, Fe+3,
Al+3. or Ti+4 in an M1 octahedral coordination site.
Z = Si+4 and Al+3 in the tetrahedral site. There is complete solid solution between Na and Ca end members and among Mg and Fe end members, with partial substitution of Al+3 for Si+4 in the tetrahedral site, and partial substitution of F for OH in the hydroxyl site. |
| The composition of the common (non-sodic) amphiboles are shown in the diagram here. Note the similarity to the pyroxene compositional diagram, above. Actinolite is the solid solution between Tremolite [Ca2Mg5Si8O22(OH)2] and Ferroactinolite [Ca2Fe5Si8O22(OH)2.] Cummingtonite - Grunerite is a solid solution between Anthophyllite [Mg7Si8O22(OH)2] and Grunerite [Fe7Si8O22(OH)2]. | 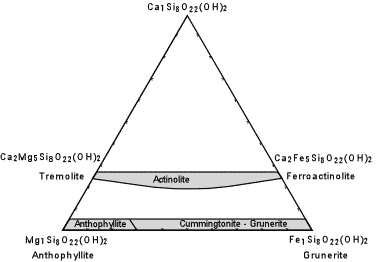 |
| Hornblende is the most common amphibole and has more in common with the
Tremolite - Ferroactinolite series, with Al substituting into the Y sites
and the tetrahedral site. It thus has the complicated formula:
(Ca,Na)2-3(Mg,Fe,Al)5Si6(Si,Al)2O22(OH,F)2 The sodic amphiboles have the following formulae: Glaucophane - Na2Mg3Al2Si8O22(OH)2 Riebeckite - Na2Fe3+2Fe2+3Si8O22(OH)2 Arfvedsonite - NaNa2Fe4+2Fe+3Si8O22(OH)2 |
| All of the amphiboles except Anthophyllite are monoclinic, and all show the excellent prismatic cleavage on {110}. The angles between the cleavages, however are 56o and 124o making all amphiboles easy to distinguish from the pyroxenes. Looking at faces that show only a single cleavage trace would show inclined extinction, except in Anthophyllite. | 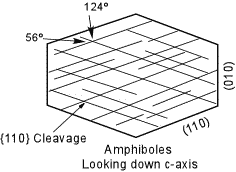 |
| Occurrence and Distinction of the Amphiboles
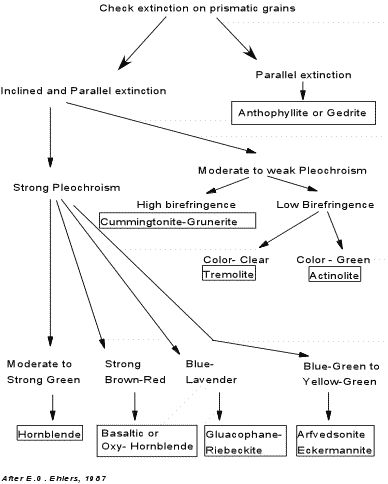
Tremolite - Occurs almost exclusively in low grade metamorphic rocks, particularly those with a high Ca concentration, such as meta-dolomites, meta-ultrabasic rocks. Tremolite in hand specimen is white in color and shows a fibrous habit and the characteristic amphibole cleavage. In thin section it is distinguished from wollastonite and diopside by its amphibole cleavage. In thin section it is clear with no pleochroism, which distinguishes it from other amphiboles. It shows high relief, inclined extinction, and is optically negative with a 2V of about 85o. Actinolite - Also occurs almost exclusively in low grade metamorphic rocks, particularly in meta-basalts and meta-gabbros where it is commonly associated with chlorite. It is green in hand specimen and shows the characteristic amphibole cleavage, usually showing an elongated habit. In thin section it shows a characteristic pale yellow to green pleochroism, has high relief, and is optically negative with a 2V of 60 to 85o. Hornblende - is a common mineral in both igneous and metamorphic rocks. In igneous rocks it is found in andesites, dacites, and rhyolites, as well as in gabbros, diorites, and granites. In metamorphic rocks it is a common constituent of meta-basalts that have been metamorphosed to intermediate grades of regional metamorphism (amphibolites). It is also found in some ultrabasic rocks. In hand specimen it is dark brown to black in color and shows the characteristic amphibole cleavage. In thin section, it shows high relief with a characteristic green - brown - yellow pleochroism. Optic sign and 2V angle cover a wide range and not very useful in the distinction of hornblende. Basaltic Hornblende (also called Oxy-hornblende)- is a dark brown to reddish brown variety of hornblende that results from oxidation during crystallization of basalts, andesites, dacites, and rhyolites. It usually has a dark reaction rim that consists of opaque oxide, and is characteristically pleochroic in yellow to brown to reddish brown colors. Anthophyllite - does not occur in igneous rocks, but is a constituent of metamorphic rocks. It is the only orthorhombic amphibole so it is easily characterized by its parallel extinction relative to the {110} cleavage. Cummingtonite - Grunerite - is more common in metamorphosed igneous rocks where members of the series occur with hornblende. It has been found in siliceous volcanic rocks as well. Cummingtonite is optically positive, while grunerite is optically negative. Members of this series can be distinguished from orthorhombic Anthophyllite by the inclined extinction of the monoclinic Cummingtonite-Grunerite series, and can be distinguished from tremolite and actinolite by the higher refractive indices and higher birefringence of the Cummingtonite Grunerite series. Glaucophane - Riebeckite - Glaucophane is a common mineral in blueschist facies metamorphic rocks that result from low temperature, high pressure metamorphism along ancient subduction zones. Riebeckite is found in alkali granites, syenites, and peralkaline rhyolites. Glaucophane is easily distinguished from the other amphiboles by its characteristic blue-lavender pleochroism. Glaucophane is length slow, whereas Riebeckite is length fast. Arfvedsonite - occurs most commonly in peralkaline volcanic rocks and alkaline plutonic igneous rocks, where it typically occurs with the sodic pyroxene aegerine. Its blue green to yellow green pleochroism distinguish it from the other amphiboles. The chart below, also found in your lab assignments, summarizes the
properties used to distinguish the amphiboles. |
 |
|
Phyllosilicates (Sheet Silicates) The phyllosilicates, or sheet silicates, are an important group of minerals that includes the micas, chlorite, serpentine, talc, and the clay minerals. |
| The basic structure of the phyllosilicates is based on interconnected six member rings of SiO4-4 tetrahedra that extend outward in infinite sheets. Three out of the 4 oxygens from each tetrahedra are shared with other tetrahedra. This leads to a basic structural unit of Si2O5-2. | 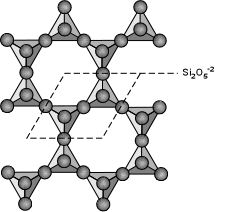 |
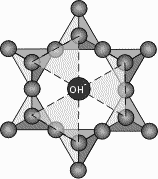 |
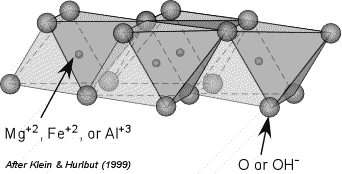
Most phyllosilicates contain hydroxyl ion, OH-, with the OH located at the center of the 6 membered rings, as shown here. Thus, the group becomes Si2O5(OH)-3. When other cations are bonded to the SiO4 sheets, they share the apical oxygens and the (OH) ions which bond to the other cations in octahedral coordination. This forms a layer of cations, usually Fe+2, Mg+2, or Al+3, that occur in octahedral coordination with the O and OH ions of the tetrahedral layer. As shown, here, the triangles become the faces of the octahedral groups that can bind to the tetrahedral layers. |
The octahedral layers take on the structure of either Brucite [Mg(OH)3] if the cations are +2 ions like Mg+2 or Fe+2, or Gibbsite [Al(OH)3] if the cations are +3 like Al+3. In the brucite structure, all octahedral sites are occupied and all of the anions are all OH-1. In the Gibbsite structure every 3rd cation site is unoccupied and all of the anions are OH-1. |
 |
This gives rise to 2 groups of sheet silicates:
We can build the structures of the various sheet silicates by starting with the octahedral layers similar to the structures of brucite or gibbsite, as discussed last semester in Earth Materials. Review your notes from the clay minerals discussion. |
| Serpentine Group
The serpentine group of minerals has the formula - Mg3Si2O5(OH)4. Three varieties of serpentine are known. Antigorite and Lizardite are usually massive and fine grained, while Chrisotile is fibrous. As discussed above, the imperfect fit of the octahedral layers and the tetrahedral layers causes the crystal structure to have to bend. |
| In Antigorite the bending of the sheets is not continuous, but occurs in sets, similar to corrugations, as shown here. | 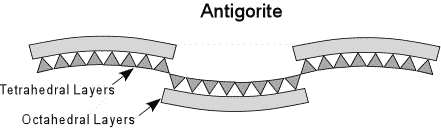 |
| In Chrisotile, the bending of the sheets is more continuous, resulting in continuous tubes that give the mineral it's fibrous habit. The Chrisotile variety is commonly referred to as asbestos. | 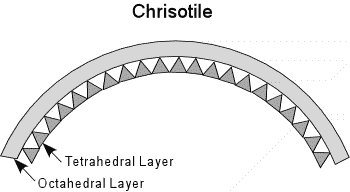 |
Occurrence - Serpentine is found as an alteration product of
Mg-rich silicates like pyroxene and olivine. It results due to
hydration. For example:
Thus, serpentine is commonly found pseudomorphed after olivines and pyroxenes in altered basic and ultrabasic igneous rocks, like altered peridotites, dunites, and sometimes basalts and gabbros. It is commonly associated with minerals like magnesite (MgCO3), chromite, and magnetite. If the rock is made up almost entirely of serpentine, it is called a serpentinite. Properties - Because the serpentines usually occur either as fine-grained aggregates or fibrous crystals, optical properties are difficult to determine. Most of the time, serpentine can be distinguished by its characteristic pseudomorphing of other crystals like olivines and pyroxenes. In hand specimen it generally tends to have a dark green color with a greasy luster. In thin section it is clear to pale green to pale yellow, but does not show pleochroism, shows a generally low relief compared to minerals like olivine and pyroxene with which it is associated, and show very low order interference colors due to its low birefringence. |
| Talc
Talc has the chemical formula - Mg3Si4O10(OH)2. It is probably best know for its low hardness. Although it has a micaceous structure, it is so easily deformed, that crystals are rarely seen. Occurrence - Like serpentine, talc requires an environment rich in Mg. It is therefore found in low grade metamorphic rocks that originated as ultrabasic to basic igneous rocks. Rocks composed almost entirely of talc have a greasy feel and are referred to as soapstone. Properties - Talc is most easily distinguished in hand specimen by its low hardness, greasy feel, and association with other Mg-bearing minerals. When crystals are present they show the characteristic micaceous cleavage on {001}. In thin section, talc is colorless, biaxial negative with a 2V of 0 to 30o. Like other sheet silicates, it shows the well developed {001} cleavage. Maximum interference colors, consistent with a birefringence of 0.05 is 3o yellow. Muscovite has a higher birefringence and higher 2V, properties which easily distinguish the 2 minerals. |
| Mica Group
The micas can be divided into the dioctahedral micas and the trioctahedral micas, as discussed above. Muscovite, Paragonite, and Margarite are the white micas, and represent the trioctahedral group, and Biotite and Clintonite (Xanthophyllite) the black or brown mica, represents the dioctahedral group. Muscovite and Biotite are the most common micas, but the Lithium- rich, pink mica, Lepidolite, K(Li,Al)2AlSi3O10(OH)2 is also common, being found mostly in pegmatites. Muscovite Muscovite, KAl3Si3O10(OH)2, and Paragonite, NaAl3Si3O10(OH)2, are two potential end members of the solid solution series involving K and Na. But, there is a large miscibility gap between the two end members with Muscovite being between 65% and 100% of K-rich end member, and Paragonite showing compositions between about 80% and 100% of the Na-rich end member. Occurrence - Muscovite is common constituent of Al-rich medium grade metamorphic rocks where is found in Al-rich schists and contributes to the schistose foliation found in these rocks. Muscovite is also found in siliceous, Al-rich plutonic igneous rocks (muscovite granites), but has not been found as a constituent of volcanic rocks. In these rocks it is commonly found in association with alkali feldspar, quartz, and sometimes biotite, garnet, andalusite, sillimanite, or kyanite. Properties - Muscovite is easily identified in hand specimen by its white to sometimes light brownish color and its perfect {001} cleavage. In thin section, the {001} cleavage is easily seen and it's high birefringence is exhibited by the large change in relief on rotation of the stage and it's 2nd to 4th order interference colors. It is clear and shows no pleochroism (which distinguishes it from Biotite), and it is biaxial negative with a 2V between 28 and 50o. One of the most diagnostic properties of the micas, including muscovite, is the mottled or birds-eye extinction exhibited by these minerals.
|
|
Biotite Biotite is a solid solution between the end members Phlogopite KMg3AlSi3O10(OH)2 and Annite KFe3AlSi3O10(OH)2, although pure Annite does not occur in nature. In addition, small amounts of Na, Rb, Cs, and Ba may substitute for K, and like in other minerals, F can substitute for OH and increase the stability of Biotite to higher temperatures and pressures. Occurrence - Nearly pure phlogopite is found in hydrous ultrabasic rocks like kimberlite, and is also found in metamorphosed dolomites. Biotite, with more Fe-rich compositions is common in dacitic, rhyolitic, and trachytic volcanic rocks, granitic plutonic rocks, and a wide variety of metamorphic rocks. In metamorphic rocks, biotite usually shows a preferred orientation with its {001} forms parallel to the schistose foliation. Properties - In hand specimen, Biotite is brown to black and shows the perfect {001} micaceous cleavage. In thin section, it shows the perfect cleavage and mottled extinction typical of all micas. It's most characteristic property is its pleochroism, showing yellow to brown to green colors. Hornblende shows similar pleochroic colors, but is distinguished from biotite by the differences in cleavage of the 2 minerals. Biotite is biaxial negative with a low 2V of 0o to 25o.
|
| Chlorite Group
As discussed above, the Chlorite group has a structure that consists of phlogopite T-O-T layers sandwiching brucite-like octahedral layer. There is substantial substitution of Mg for Fe, and Al can substitute for (Mg, Fe) in both the octahedral sites, as well as for Si in the tetrahedral sites. Thus, chlorite can have a rather complicated formula - (Mg,Fe,Al)3(Si,Al)4O10(OH)6. Occurrence- Chlorite is a common mineral in low grade metamorphic rocks, where it occurs in association with minerals like actinolite, epidote, and biotite. It also forms as an alteration product of pyroxenes, amphiboles, biotite, and garnet in igneous as well a metamorphic rocks. Properties - In hand specimen, chlorite is recognized by its green color, micaceous habit and cleavage, and association with other minerals like actinolite and epidote. In thin section, Chlorite shows low relief and low birefringence, with a characteristic midnight blue to black anomalous interference color. It shows some pleochroism in the range of green to pale yellow. It is easily distinguished from biotite by its lower relief and anomalous interference color. |
|
|