Peptide Structural Analysis Exercise
This exercise will illustrate practical aspects of polypeptide
structural
analysis including solubility, conformational averaging, and proton NMR
resonance assignment.
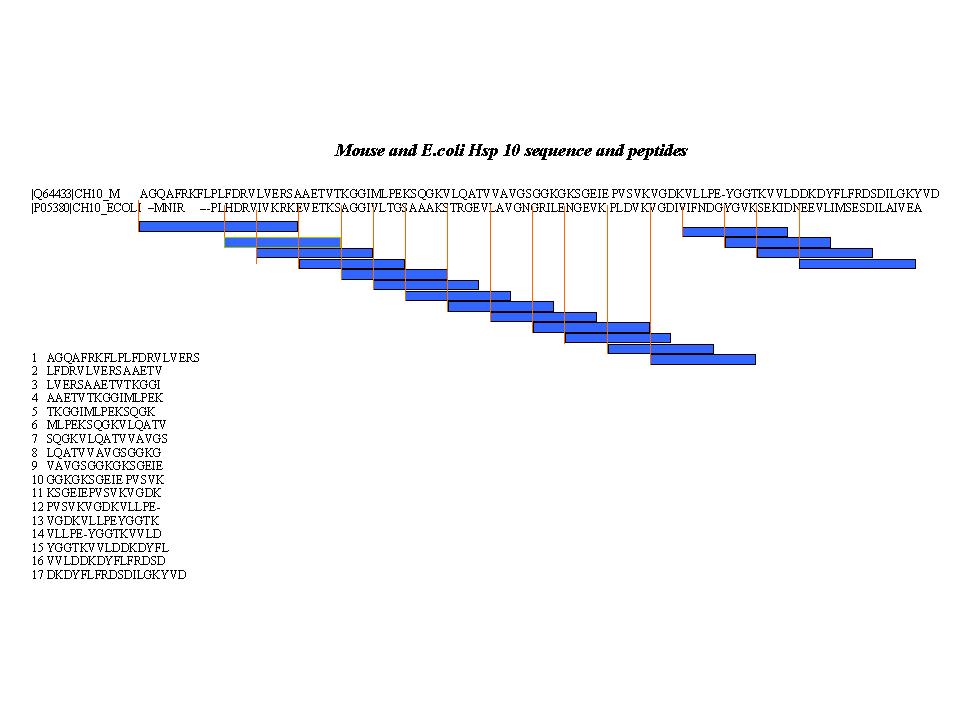
Subject: A 15-residue peptide corresponding to one of seventeen
overlapping
segments of the human Hsp10 (left-overs from Dr. Landry's immunology
project,
see below).
Tasks
- Peptide solubilization - Weigh out approximately 1 mg peptide and
attempt
to dissolve it in 50 microliters of water (final concentration,
approximately
2 mM); failing that, then add 10-30% hexafluoroisopropanol
(HFIP); failing
that,
then prepare a sample in DMSO. [Schedule approximately 1 hour
with
Landry-lab analytical balance and pipettemen?] Check sample pH
using
indicator paper.
- Conformational analysis by CD - Obtain far-UV CD spectra of 50
micromolar
samples in 10 mM phosphate buffer, buffer plus 10% HFIP, buffer plus
20% HFIP, and buffer plus 30% HFIP. [Schedule
1 hour with instructor or designee and CD spectropolarimeter]
- Prepare 0.7 ml NMR sample of 2 mM peptide in buffer plus 30%
HFIP-d2
(D, 98%). Obtain 2D proton NMR spectra for assignment of backbone
resonances, nuclear Overhauser effect (NOESY) and total correlated
(TOCSY)
spectra. Students will be encouraged to observe data acquisition
and processing by the instructor or NMR facility manager. All
spectra
should be completed by mid-semester. Data plotting and assignment
to be carried out by students using the PC called MrBlack.
- Project report - CD spectra with interpretation (fraction alpha
helix),
table of backbone proton NMR assignments and annotated fingerprint
(NH-alpha)
and NH-upfield regions of the TOCSY spectrum with interpretation
(conformational
behavior, e.g., deviations from random coil values)
Each peptide has a high likelihood of solubility in water. The
peptides
are expected to be random coils in water but have varying degrees of
alpha
helix in HFIP. Some peptides have as many as three of the same
residue
type, thus necessitating methods of sequential assignment.
Several
peptides have proline, which will exist in cis and trans
conformers.
Thus, two sets of resonances will be present for several residues
surrounding
the proline. This is a good illustration of conformational
heterogeneity.
Since these are overlapping peptides, some subsequences can be shared
by
two peptides, potentially revealing effects of context on chemical
shift.
Results will be exchanged among students and discussed in class.
GBCH722
Home