Effusive (Non-explosive) Eruptions
Non explosive eruptions are favored by low gas content and low viscosity magmas (basaltic
to andesitic magmas). If the viscosity is low, non-explosive eruptions usually begin with fire fountains due to
release of dissolved gases.
When magma reaches the surface of the earth, it is called lava. Since it its a liquid, it flows downhill in response to gravity as a lava flows. Different magma types behave differently as lava flows, depending on their temperature, viscosity, and gas content.
Lava Flows
Pahoehoe Flows - Basaltic lava flows with low viscosity start to cool when exposed to the low temperature of the atmosphere. This causes a surface skin to form, although it is still very hot and behaves in a plastic fashion, capable of deformation. Such lava flows that initially have a smooth surface are called pahoehoe flows. Initially the surface skin is smooth, but often inflates with molten lava and expands to form pahoehoe toes or rolls to form ropey pahoehoe. (See figure 6.17 in your text). Pahoehoe flows tend to be thin and, because of their low viscosity travel long distances from the vent.
A'A' Flows - Higher viscosity basaltic and andesitic lavas also initially develop a smooth surface skin, but this is quickly broken up by flow of the molten lava within and by gases that continue to escape from the lava. This creates a rough, clinkery surface that is characteristic of an A'A' flow (see figure 6.18 in your text).
Pillow Lavas - When lava erupts on the sea floor or other body of water, the surface skin forms rapidly, and, like with pahoehoe toes inflates with molten lava. Eventually these inflated balloons of magma drop off and stack up like a pile of pillows and are called pillow lavas. Ancient pillow lavas are readily recognizable because of their shape, their glassy margins and radial fractures that formed during cooling.
Siliceous Lava Flows - High viscosity andesitic and rhyolitic lava flows, because they can’t flow very easily, form thick stubby flows that don’t move far from the vent.
Lava Domes or Volcanic Domes - result from the extrusion of highly viscous, gas poor andesitic and rhyolitic lava. Since the viscosity is so high, the lava does not flow away from the vent, but instead piles up over the vent. Blocks of nearly solid lava break off the outer surface of the dome and roll down its flanks to form a breccia around the margins of domes. The surface of volcanic domes are generally very rough, with numerous spines that have been pushed up by the magma from below.
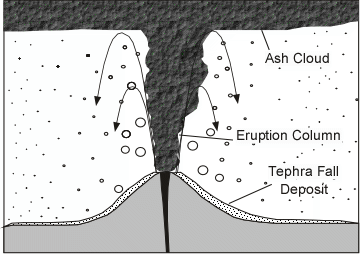
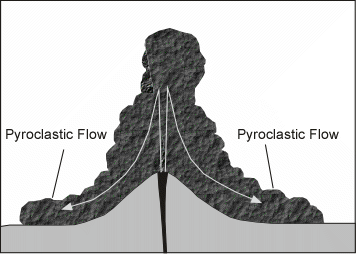
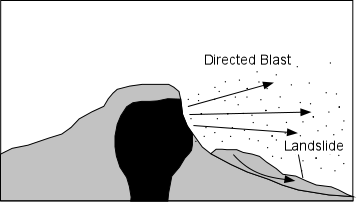
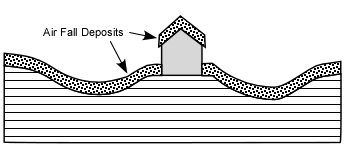
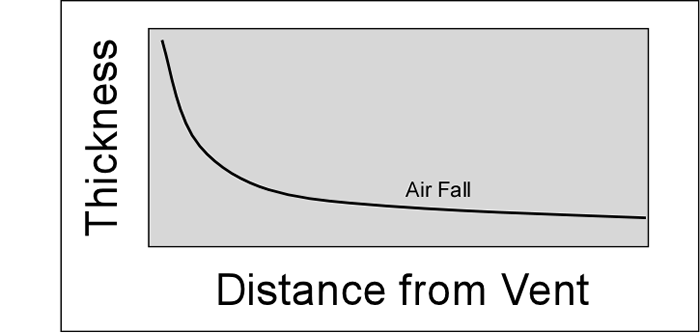
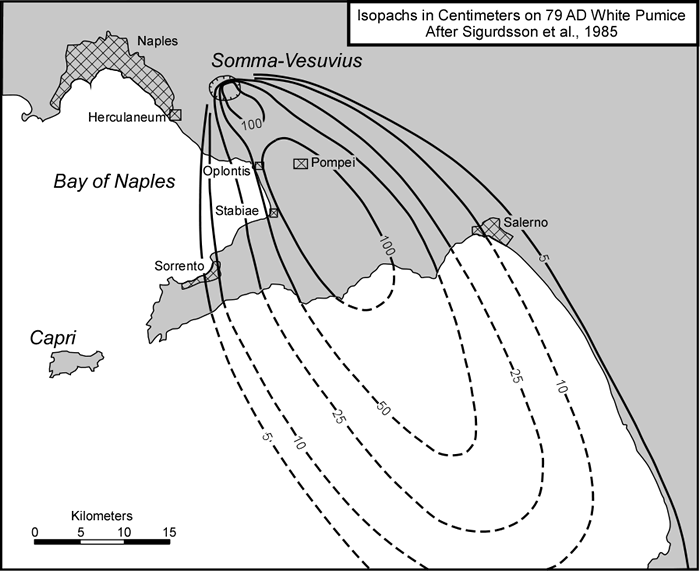
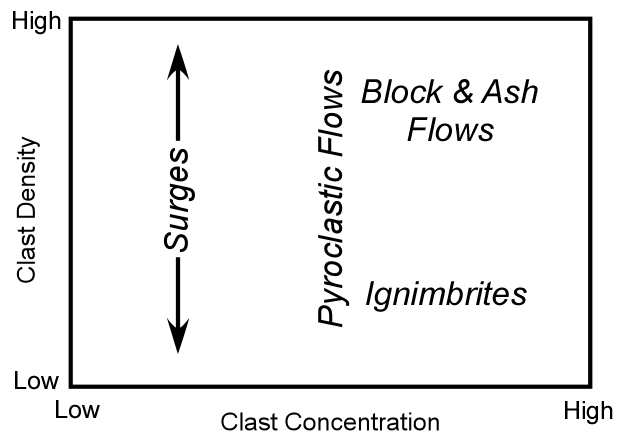
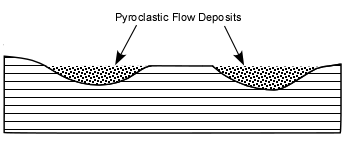
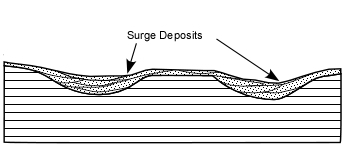
Explosive Eruptions
Explosive eruptions are favored by high gas content and high viscosity (andesitic to
rhyolitic magmas). Explosive bursting of bubbles will fragment the magma into clots of liquid that will
cool as they fall through the air. These solid particles become pyroclasts
(meaning - hot fragments) and tephra or volcanic
ash, which refer to sand- sized or smaller
fragments.
|