Types of bonding:
- Ionic Bonds - caused by the force of attraction between ions of opposite charge.
Example Na+1 and Cl-1. Bond to form NaCl (halite or salt).
Ionic bonds are moderately strong.
EENS1110 |
Physical Geology |
| Tulane University | Prof. Stephen A. Nelson |
Minerals |
|
|
|
|
The Earth is composed of rocks. Rocks are aggregates of
minerals. So minerals are the basic building blocks of the Earth. Currently there are over 4,000 different minerals known and dozens of new minerals are discovered each year. Our society depends on minerals as sources of metals, like Iron (Fe), Copper (Cu), Gold (Au), Silver (Ag), Zinc (Zn), Nickel (Ni), and Aluminum (Al), etc., and non-metals such as gypsum, limestone, halite, clay, and talc. Many minerals of of great economic importance and their distribution, extraction, and availability have played an important role in history. Minerals are composed of atoms. We'll start our discussion with the geological definition of a Mineral. A mineral is
A mineraloid is a substance that satisfies some, but not all of the parts of the definition. For example, opal, does not have a characteristic crystalline structure, so it is considered a mineraloid. Note also that the "minerals" as used in the nutritional sense are not minerals as defined geologically. Examples
Atoms Since minerals (in fact all matter) are made up of atoms, we must first review atoms. Atoms make up the chemical elements. Each chemical element has nearly identical atoms. An atom is composed of three different particles:
Each element has the same number of protons and the same number of electrons.
Isotopes are atoms of the same element with differing numbers of neutrons. i.e. the number of neutrons may vary within atoms of the same element. Some isotopes are unstable which results in radioactivity.
|
| Structure of Atoms
The drive to attain a stable electronic configuration in the outermost shell along with the fact that this sometimes produces oppositely charged ions, results in the binding of atoms together. When atoms become attached to one another, we say that they are bonded together. |
Types of bonding:
|
|
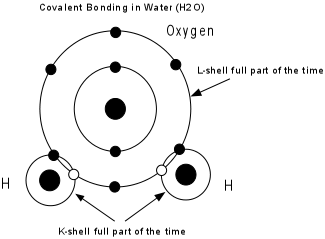 |
Several different bond types can be present in a mineral, and these determine the physical properties of the mineral. |
|
All minerals, by definition are also crystals. Packing of atoms in a crystal structure requires an orderly and repeated atomic arrangement. Such an orderly arrangement needs to fill space efficiently and keep a charge balance. Since the size of atoms depends largely on the number of electrons, atoms of different elements have different sizes. Example of NaCl : For each Na atom there is one Cl atom. Each Na is surrounded by Cl and each Cl is surrounded by Na. The charge on each Cl is -1 and the charge on each Na is +1 to give a charged balanced crystal. The structure of minerals is often seen in the shape of crystals. The law of constancy of interfacial angles --- Angles between the same faces on crystals of the same substance are equal. This is a reflection of ordered crystal structure (See figure 5.5 in the textbook). Crystal structure can be determined by the use of X-rays. A beam of X-rays can penetrate crystals but is deflected by the atoms that make up the crystals. The image produced and collected on film, can be used to determine the struture. The method is know as X-ray diffraction. Crystal structure depends on the conditions under which the mineral forms. Polymorphs are minerals with the same chemical composition but different crystal structures. The conditions are such things as temperature (T) and pressure (P), because these effect ionic radii. At high T atoms vibrate more, and thus distances between them get larger. Crystal structure changes to accommodate the larger atoms. At even higher T substances changes to liquid and eventually to gas. Liquids and gases do not have an ordered crystal structure and are not minerals. Increase in P pushes atoms closer together. This makes for a more densely packed
crystal structure. |
|
Examples:
|
|
|
Ionic Substitution (Solid Solution) Ionic substitution - (also called solid solution), occurs because some elements (ions) have the same size and charge, and can thus substitute for one another in a crystal structure. Examples:
Composition of Minerals The variety of minerals we see depend on the chemical elements available to form them. In the Earth's crust the most abundant elements are as follows:
Note that Carbon (one of the most abundant elements in life) is not among the top 12. Properties of Minerals Physical properties of minerals allow us to distinguish between minerals and thus identify them, as you will learn in lab. Among the common properties used are:
|
|
Formation of Minerals Minerals are formed in nature by a variety of processes. Among them are:
Since each process leads to different minerals and different mineral polymorphs, we can identify the process by which minerals form in nature. Each process has specific temperature and pressure conditions that can be determined from laboratory experiments. Example: graphite and diamond, as shown previously. Rocks - Mixtures of Minerals Mixtures or aggregates of minerals are called rocks. There are three basic kinds of rocks, each type is determined by the process by which the rock forms.
Each of these rock forming processes results in distinctive mineral assemblages and textures in the resulting rock. Thus, the different mineral assemblages and textures give us clues to how the rock formed. An understanding of the rock forming processes and the resulting mineral assemblage and texture will be the main goal of the next part of this course.
Questions on this material that could be asked on an exam.
|