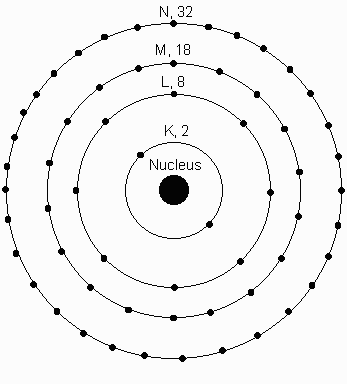
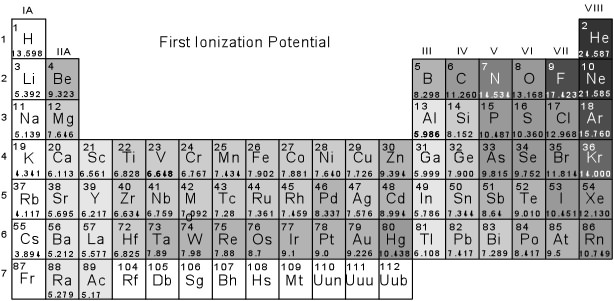
- The Noble gases all have very high first ionization potentials,
indicating that their electronic structure is stable. A glance
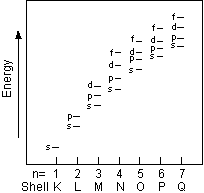
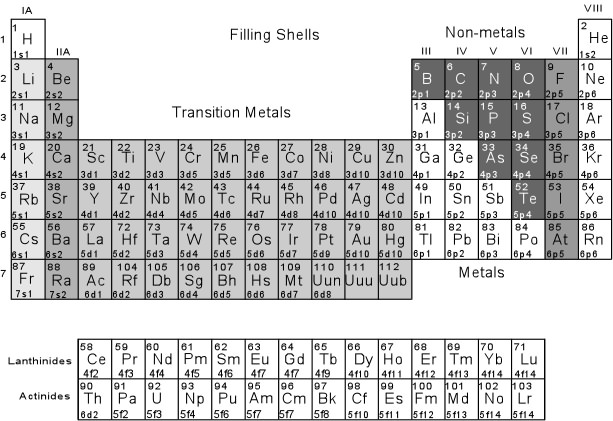
at the periodic table showing filling shells (above) indicates that
the Noble Gases all have in common completely filled p - orbitals.
It is because these sub-orbital shells are full that these elements do
not readily become ions and do not easily combine with other elements
to become compounds.
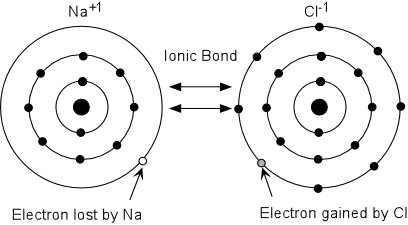
- Elements in Group I (the alkalies), on the other hand have very low
first ionization potentials, and thus it is relatively easy to remove
one electron. Since all of these elements have in common an
outermost shell containing 1 electron in the s - orbital, these
elements tend to become +1 ions (i.e. Li+1, Na+1,
K+1, Rb+1, Cs+1, etc.) Note that
removal of this electron will leave the atoms with an electronic
configuration of the Noble gases, i.e. they will have completely
filled outermost electron shells. Second ionization potentials
(the energy required to remove a second electron) is also very high
for these elements, again indicating that once they become +1 ions
they have a stable electronic configuration.
- Elements in Group II (the alkaline earths) also have relatively low
first ionization potentials and have relatively low second ionization
potentials. Thus, these elements tend to lose 2 electrons to
become +2 ions (i.e. Be+2, Mg+2, Ca+2,
Sr+2, Ba+2, etc.) Once they have lost
these two electrons they also have an electronic configuration with
completely filled outer electron shells, similar to the Noble Gases.
- Elements in Group VII (the halogens) have very high first ionization
potentials. They don't like to give up electrons. But note
that if they gain an electron to become a -1 ion, they will also have
completely filled outer electron shells similar to the Noble
gases. Thus these elements tend to gain electrons to become -1
ions (i.e. F-1, Cl-1, Br-1, etc.).
- Based on similar reasoning, Group III elements tend to lose 3
electrons to become +3 ions (i.e. B+3, Al+3, Ga+3,
etc.). Group IV elements tend to lose 4 electrons to become +4
ions (i.e. C+4, Si+4, Ge+4).
But Pb, usually only loses 2 electrons to become Pb+2.
- Elements in Group V tend to lose 5 electrons to become +5 ions (i.e.
N+5, P+5, As+5).
- Group VI tend to gain electrons to become -2 ions (i.e. O-2,
S-2, Se-2), but sulfur sometimes loses 6
electrons to become S+6.
- The transition elements all have d-orbital electrons in their
outermost shells, and because they have low to high first ionization
potentials their behavior is somewhat variable. The elements in
the third column tend to become +3 ions (Sc+3, Y+3,
La+3 ), those in the 4th column tend to become +4 ions (Ti+4,
Zr+4, Hf+4), and those in the 5th column tend to
become +5 ions (V+5, Nb+5, Ta+5).
But the 5th through 11th column show variable ions. For example,
Cr is usually Cr+3, Mn can be usually Mn+2,
Mn+3, or Mn+4, Fe can be either Fe+2
(ferrous iron) or Fe+3 (ferric iron), Ni, Co, and Zn
become +2 ions, and Cu can be either Cu+1or Cu+2.
- The rare earth elements tend to become +3 ions, with the exception
of Eu, which can be either Eu+2, or Eu+3.
The actinides U and Th tend to become +4 ions.
To summarize, the common valence states of the common elements are
listed in the table below for quick reference.
|