
| EENS 2110 |
Mineralogy |
| Tulane University |
Prof. Stephen A. Nelson |
|
Crystal Morphology, Crystal Symmetry, Crystallographic Axes |
|
|
|
|
Crystal Morphology and Crystal Symmetry
The symmetry observed in crystals as exhibited by their
crystal faces is due to the ordered internal arrangement of atoms in a
crystal structure, as mentioned previously. This arrangement of
atoms in crystals is called a lattice. |
| In 2-dimensions a plane lattice consists of an orderly array
of points. The array is defined by the spacing between points and the directions
(or angles) between the points. Thus, the array can be reproduced by
specifying the distance and angle to move from point to point. This
is referred to as translational symmetry. In the
example here, the array is reproduced by moving down a distance a and
moving to the right a distance b. The angle between the two
directions of translation in this case is 90o
|
 |
| In the example to the right, the translation distances a and b are not equal and the translation angle is not 90o. | 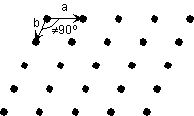 |
| Crystals, of course, are made up of 3-dimensional arrays of atoms.
Such 3-dimensional arrays are called space lattices. We discuss these space lattices in 3-dimensions in much more detail
later. For now, however, we will continue to look a plane lattices
and note that everything that applies to these 2-dimensional lattice also
applies to space lattices.
There are four important points about crystal lattices that are noteworthy for our study of crystals:
|
|
 |
|
|
 |
|
 |
|
|
 |
| In order to know which faces on different crystals are the corresponding
faces, we need some kind of standard coordinate system onto which we can
orient the crystals and thus be able to refer to different directions and
different planes within the crystals. Such a coordinate system is
based on the concept of the crystallographic axes. |
|
The crystallographic axes are imaginary lines that we can draw within the crystal lattice. These will define a coordinate system within the crystal. For 3-dimensional space lattices we need 3 or in some cases 4 crystallographic axes that define directions within the crystal lattices. Depending on the symmetry of the lattice, the directions may or may not be perpendicular to one another, and the divisions along the coordinate axes may or may not be equal along the axes. As we will see later, the lengths of the axes are in some way proportional to the lattice spacing along an axis and this is defined by the smallest group of points necessary to allow for translational symmetry to reproduce the lattice. We here discuss the basic concepts of the crystallographic axes. As we will see, the axes are defined based on the symmetry of the lattice and the crystal. Each crystal system has different conventions that define the orientation of the axes, and the relative lengths of the axes. |
|
|
| Unit Cells
The "lengths" of the various crystallographic axes are defined on the basis of the unit cell. When arrays of atoms or molecules are laid out in a space lattice we define a group of such atoms as the unit cell. This unit cell contains all the necessary points on the lattice that can be translated to repeat itself in an infinite array. In other words, the unit cell defines the basic building blocks of the crystal, and the entire crystal is made up of repeatedly translated unit cells. |
In defining a unit cell for a crystal the choice is somewhat
arbitrary. But, the best choice is one where:
|
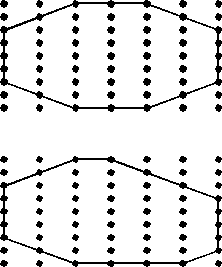
For example, in the 2-dimensional lattice shown here there are 6 possible choices to define the unit cell, labeled a through f. The lattice has 2-fold rotational symmetry about an axis perpendicular to the page. Since the lattice itself does not have 3-fold or 6-fold rotational symmetry, choices a and b would not be wise choices for the unit cell. Choice f can be eliminated because it is really just half of cell b. The edges of c and e are not coincident or parallel to any 2-fold axes that lie in the plane of the page. Thus our best choice would cell d. |
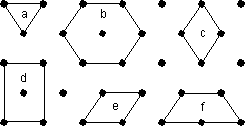 |
| Once we have chosen a unit cell for the crystal, then it can be oriented
on the crystallographic axes to define the angles between the axes and to
define the axial lengths. This will allow us to define directions
within the crystal that become important when we realize that many
properties of crystals depend on direction in the crystal.
Properties that depend on direction in the crystal are called vectorial
properties. We'll discuss these in a later lecture.
Another important point is that the relative lengths of the crystallographic axes, or unit cell edges, can be determined from measurements of the angles between crystal faces. We will consider measurements of axial lengths, and develop a system to define directions and label crystal faces in the next lecture. |
Examples of questions on this material that could be asked on an exam
|