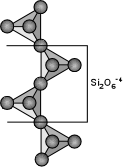
XYZ2O6
| EENS 2110 |
Mineralogy |
| Tulane University |
Prof. Stephen A. Nelson |
|
Inosilicates (Pyroxenes and Amphiboles) |
|
|
|
|
Inosilicates (Single Chain Silicates) |
The single chain silicates have a basic structural unit consisting of
linked SiO4 tetrahedra that each share 2 of their oxygens in
such a way as to build long chains of SiO4. The basic
structural group is thus Si2O6 with an Si:O ratio
of 1:3. The most important inosilicates are the pyroxenes.
These have a general structural formula of:
|
 |
|
where X = Na+, Ca+2, Mn+2, Fe+2, or Mg+2 filling octahedral sites called M2
The pyroxenes can be divided into several groups based on chemistry and crystallography: Orthorhombic Pyroxenes (Orthopyroxenes - Opx)
Monoclinic Pyroxenes (Clinopyroxenes - Cpx) The Diopside- Hedenbergite series - Diopside (CaMgSi2O6) - Ferrohedenbergite (CaFeSi2O6) The Sodic Pyroxenes - Jadeite (NaAlSi2O6) and Aegerine (NaFe+3Si2O6) Augite is closely related to the diopside - Hedenbergite series with addition of Al and minor Na substitution - (Ca,Na)(Mg,Fe,Al)(Si,Al)2O6 Pigeonite is also a monoclinic pyroxene with a composition similar to the orthopyroxenes with more Ca substituting for Fe, and Mg. |
| The compositional range of the Ca-rich, Al-free pyroxenes in shown in the triangular composition diagram here. Note that there is complete Mg-Fe substitution and small amounts of Ca substitution into the Orthopyroxene solid solution series. Mg-rich varieties of orthopyroxene are called hypersthene, whereas Fe-rich varieties are called Ferrosilite. There is also complete Mg-Fe solid solution between Diopside and Ferrohedenbergite, with some depletion in Ca. CaSiO3 is the chemical formula for wollastonite, but wollastonite does not have a pyroxene structure. | 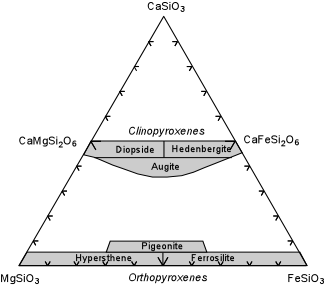 |
|
 |
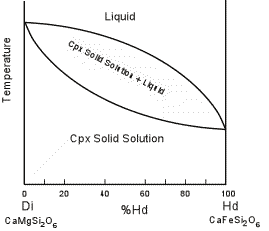 |
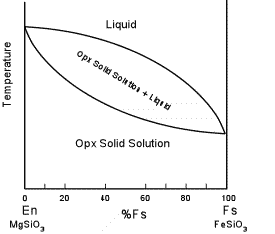
| Solid immiscibility is present between the Diopside - Hedenbergite
series and the Orthopyroxene series. This is seen in the phase
diagram below which shows a hypothetical phase diagram running from the
orthopyroxenes to the clinopyroxenes. Note the solvi.
Pigeonite is only stable at higher temperatures and inverts to
orthopyroxene if cooled slowly to lower temperatures. Thus,
pigeonite is only found in volcanic and shallow intrusive igneous rocks,
or as exsolution lamellae in a host augite or opx (more commonly in
augite). |
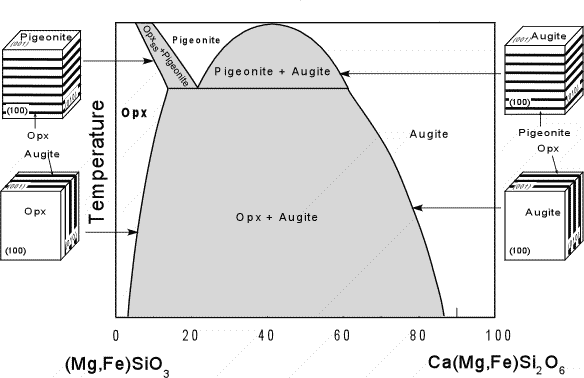 |
When pigeonite or augite exsolve they may form exsolution lamellae that form parallel to the (001) plane. At lower temperature the exsolution of Opx or augite result in exsolution lamellae that are parallel to the (100) plane. |
All pyroxenes show perfect {110} cleavage. When viewed looking down the c-crystallographic axis, the cleavages intersect at near 90o angles (the angles are actually 92 - 93o and 87 - 880). This 90 degree cleavage angle is most useful in distinguishing pyroxenes from amphiboles (in amphiboles the cleavages are at 56o and 124o. |
|
| Distinguishing Opx from Cpx in thin section is accomplished by noting that in all orthorhombic pyroxenes the prismatic {110} cleavage will show parallel extinction. If looking down the c-axis the extinction will be symmetrical relative to the two cleavage traces. |
|
| In Cpx, however, one would see inclined extinction on all faces except {100}. Thus, one should check several grains for extinction before concluding that the mineral is Opx, since there is always a slight chance that one is looking at a {100} face. Note that in Cpx, the maximum extinction angle will only be observed if one is looking at a {010} face. |
|
| Occurrence and Distinction of the Pyroxenes
Augite - is commonly found in both plutonic and volcanic igneous rocks, as well as high grade meta-igneous rocks like gneisses and granulites. It is easily distinguished from amphiboles by the nearly 90ocleavage angles, and is distinguished from Opx by inclined extinction relative to the {110} cleavage, as discussed above. Augite also has higher maximum birefringence than Opx, and shows 2nd to 3rd order interference colors. Augite is optically positive with a 2V of about 60o. It shows high relief, relative to quartz and feldspars and is commonly colorless to brown or green in thin section, showing no pleochroism. Hypersthene - is commonly found in both plutonic and volcanic igneous rocks and in meta-igneous rocks as well. It is distinguished from augite by its lower interference colors and lack of inclined extinction relative to {110}. Hypersthene is sometimes pleochroic, showing light pink to light green colors. The chemical composition of hypersthene can be estimated using 2V (see p. 163 of DHZ). Compositions close to Enstatite are optically positive with a 2V of 60 to 90o, whereas intermediate compositions are optically negative with a 2V of 50 to 90o. Pigeonite - is generally only found in volcanic igneous rocks, although, as mentioned above, it can occur as exsolution lamellae in augites of more slowly cooled igneous rocks. Pigeonite is distinguished from augite by its lower 2V of 0 to 30o, and is distinguished from hypersthene by its lack of pleochroism, lower 2V and inclined extinction relative to the {110} cleavage. Aegerine (acmite) - Aegerine Augite - are sodic pyroxenes and thus are found in alkalic igneous rocks associated with sodic amphiboles, alkali feldspars, and nepheline. The mineral is common in alkali granites, quartz syenites, and nepheline syenites (all alkalic plutonic rocks), and are also found in sodic volcanic rocks like peralkaline rhyolites. |
|
Aegerine is distinguished from other clinopyroxenes by a low extinction angle relative to the {110} cleavage (0 -10o, with augite having an extinction angle of 35 - 48o), and by the green brown pleochroism present in aegerine. Aegerine is also optically negative with a 2V of 60 to 70o, whereas Aegerine-augite has a higher 2V and can be optically positive or negative. It is distinguished from the pleochroic sodic amphiboles by its nearly 90o pyroxene cleavage angle. |
|
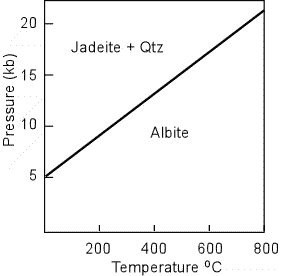
Jadeite - is a sodium aluminum pyroxene that is characterized by its presence in metamorphic rocks formed at relatively high pressure. It can form by a reaction of Albite to produce : NaAlSi3O8 = NaAlSi2O6
+ SiO2 Jadeite has a lower refractive index than all other pyroxenes, and has low birefringence, showing low order 1st and 2nd order interference colors. |
 |
|
It is monoclinic with an extinction angle of 33 to 40o,
and can thus be easily distinguished form hypersthene. It is
usually colorless in thin section, helping to distinguish it from augite
and aegerine, and has lower birefringence than augite and aegerine. |
|
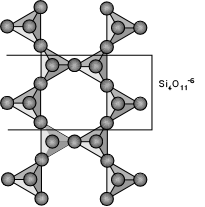
Inosilicates (Double Chain Silicates) - The Amphiboles The amphibole group of minerals is based on the double-chain silicate structure as shown here. The basic structural unit is (Si4O11)-6. The structural formula can be written as: |
W0-1X2Y5Z8022(OH,F)2 where W = Na+1 or K+1 in the A site with 10 to 12 fold coordination. X = Ca+2, Na+1, Mn+2, Fe+2, Mg+2, Fe+3, in an M4 site with 6 to 8 fold coordination. |
 |
Y = Mn+2, Fe+2, Mg+2, Fe+3, Al+3. or Ti+4 in an M1 octahedral coordination site. Z = Si+4 and Al+3 in the tetrahedral site. There is complete solid solution between Na and Ca end members and among Mg and Fe end members, with partial substitution of Al+3 for Si+4 in the tetrahedral site, and partial substitution of F for OH in the hydroxyl site. |
| The composition of the common (non-sodic) amphiboles are shown in the diagram here. Note the similarity to the pyroxene compositional diagram, above. Actinolite is the solid solution between Tremolite [Ca2Mg5Si8O22(OH)2] and Ferroactinolite [Ca2Fe5Si8O22(OH)2.] Cummingtonite - Grunerite is a solid solution between Anthophyllite [Mg7Si8O22(OH)2] and Grunerite [Fe7Si8O22(OH)2]. | 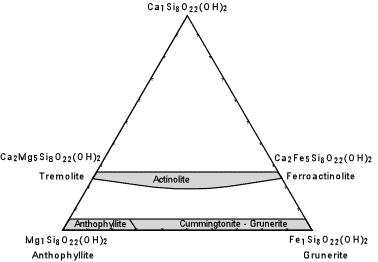 |
| Hornblende is the most common amphibole and has more in common with the
Tremolite - Ferroactinolite series, with Al substituting into the Y sites
and the tetrahedral site. It thus has the complicated formula:
(Ca,Na)2-3(Mg,Fe,Al)5Si6(Si,Al)2O22(OH,F)2 The sodic amphiboles have the following formulae: Glaucophane - Na2Mg3Al2Si8O22(OH)2 Riebeckite - Na2Fe3+2Fe2+3Si8O22(OH)2 Arfvedsonite - NaNa2Fe4+2Fe+3Si8O22(OH)2 |
| All of the amphiboles except Anthophyllite are monoclinic, and all show the excellent prismatic cleavage on {110}. The angles between the cleavages, however are 56o and 124o making all amphiboles easy to distinguish from the pyroxenes. Looking at faces that show only a single cleavage trace would show inclined extinction, except in Anthophyllite. | 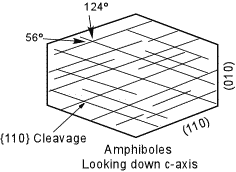 |
| Occurrence and Distinction of the Amphiboles
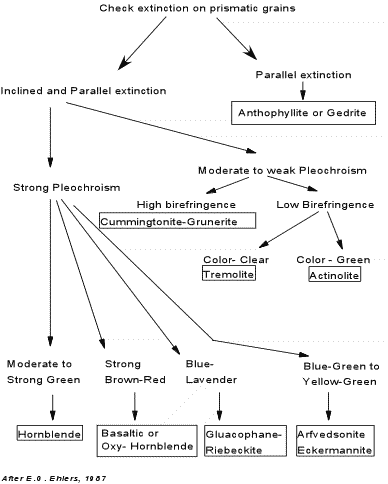
Tremolite - Occurs almost exclusively in low grade metamorphic rocks, particularly those with a high Ca concentration, such as meta-dolomites, meta-ultrabasic rocks. Tremolite in hand specimen is white in color and shows a fibrous habit and the characteristic amphibole cleavage. In thin section it is distinguished from wollastonite and diopside by its amphibole cleavage. In thin section it is clear with no pleochroism, which distinguishes it from other amphiboles. It shows high relief, inclined extinction, and is optically negative with a 2V of about 85o. Actinolite - Also occurs almost exclusively in low grade metamorphic rocks, particularly in meta-basalts and meta-gabbros where it is commonly associated with chlorite. It is green in hand specimen and shows the characteristic amphibole cleavage, usually showing an elongated habit. In thin section it shows a characteristic pale yellow to green pleochroism, has high relief, and is optically negative with a 2V of 60 to 85o. Hornblende - is a common mineral in both igneous and metamorphic rocks. In igneous rocks it is found in andesites, dacites, and rhyolites, as well as in gabbros, diorites, and granites. In metamorphic rocks it is a common constituent of meta-basalts that have been metamorphosed to intermediate grades of regional metamorphism (amphibolites). It is also found in some ultrabasic rocks. In hand specimen it is dark brown to black in color and shows the characteristic amphibole cleavage. In thin section, it shows high relief with a characteristic green - brown - yellow pleochroism. Optic sign and 2V angle cover a wide range and not very useful in the distinction of hornblende. Basaltic Hornblende (also called Oxy-hornblende)- is a dark brown to reddish brown variety of hornblende that results from oxidation during crystallization of basalts, andesites, dacites, and rhyolites. It usually has a dark reaction rim that consists of opaque oxide, and is characteristically pleochroic in yellow to brown to reddish brown colors. Anthophyllite - does not occur in igneous rocks, but is a constituent of metamorphic rocks. It is the only orthorhombic amphibole so it is easily characterized by its parallel extinction relative to the {110} cleavage. Cummingtonite - Grunerite - is more common in metamorphosed igneous rocks where members of the series occur with hornblende. It has been found in siliceous volcanic rocks as well. Cummingtonite is optically positive, while grunerite is optically negative. Members of this series can be distinguished from orthorhombic Anthophyllite by the inclined extinction of the monoclinic Cummingtonite-Grunerite series, and can be distinguished from tremolite and actinolite by the higher refractive indices and higher birefringence of the Cummingtonite Grunerite series. Glaucophane - Riebeckite - Glaucophane is a common mineral in blueschist facies metamorphic rocks that result from low temperature, high pressure metamorphism along ancient subduction zones. Riebeckite is found in alkali granites, syenites, and peralkaline rhyolites. Glaucophane is easily distinguished from the other amphiboles by its characteristic blue-lavender pleochroism. Glaucophane is length slow, whereas Riebeckite is length fast. Arfvedsonite - occurs most commonly in peralkaline volcanic rocks and alkaline plutonic igneous rocks, where it typically occurs with the sodic pyroxene aegerine. Its blue green to yellow green pleochroism distinguish it from the other amphiboles. The chart below, also found in your lab assignments, summarizes the properties used to distinguish the amphiboles. |
 |
Examples of questions on this material that could be asked on an exam
|