| Prof. Stephen A. Nelson |
EENS 211 |
| Tulane University |
Mineralogy |
|
The Isotropic Indicatrix, Isotropic Minerals, and the Immersion Method |
|
|
|
| As discussed in the last lecture, isotropic substance are those wherein
the velocity of light or the refractive index does not vary with direction
in the substance. Substances such as gases, liquids, glasses,
and minerals that crystallize in the isometric crystal system are
isotropic. We here introduce the concept of the optical
indicatrix then look at what we see when we look at isotropic substances
through the polarizing microscope. We then see how to determine the
refractive index of isotropic substances as a means to identify them, and
then take a first look at uniaxial materials.
The Isotropic Indicatrix The concept of the optical indicatrix is important as a visual means of looking at the way refractive index varies with direction in a substance. For isotropic minerals and substances the indicatrix is pretty trivial, since the refractive index does not vary with direction. |
| The optical indicatrix is simply a three dimensional object constructed by drawing vectors of length proportional to the refractive index for light vibrating parallel to the vector direction from a central point. The ends of all of the vectors are then connected to form the indicatrix. For isotropic minerals the indicatrix is a sphere as can be seen here. The indicatrix can be placed anywhere within or on a crystal so long as the crystallographic directions in the indicatrix are moved parallel to themselves. Again, for the isotropic indicatrix, this is fairly trivial since the refractive indices do not correspond to crystallographic directions and the refractive indices are the same in all directions, but the usefulness of the indicatrix concept will become much more clear when we look at anisotropic substances. |  |
|
Isotropic Substances and Polarized Light As discussed last time, the polarizing microscope has two polarizers. The lower polarizer (often just called the polarizer) is above the light source, and thus creates polarized light that vibrates in the East West direction. The upper polarizer, called the analyzer, is polarized to create polarized light vibrating at 90o to that produced by the lower polarizer. Thus, if there is only air, an isotropic substance, between the two polarizers, the E-W vibrating light is completely eliminated at the analyzer, and no light passes through the ocular lens. Isotropic substances do not change the vibration direction of light as the light passes through the substance. |
| So, if we place a mineral grain on a glass slide (glass is also
isotropic), and view the grain through the ocular lens with the analyzer
not inserted in the light path, we will be able to clearly see the
grain. If the grain selectively absorbs light of certain
wavelengths, then the grain will show its absorption
color.
If we now insert the analyzer in the light path, the light coming out of the grain will still be polarized in an E-W direction, since isotropic substances do not change the polarization direction, and the analyzer will cut out all of this light. Thus, no light coming out of the mineral grain will pass through the analyzer. The mineral is thus said to be extinct in this position. Similarly, if we rotate the stage of the microscope, and thus rotate the grain, it will remain extinct for all rotation positions. (of course this is also true for the glass slide on which the grain rests, since glass is also isotropic). |
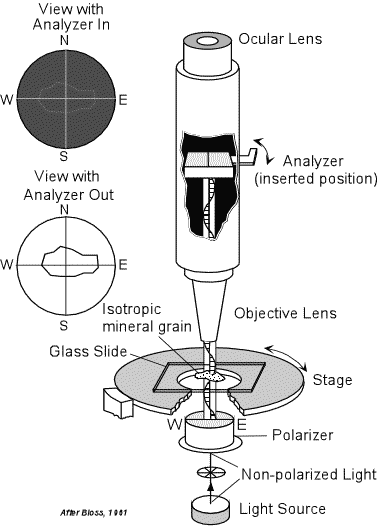 |
| This is the primary means to determine whether or not a substance is isotropic. That is, rotate the grain on the microscope stage with the analyzer inserted. If the grain remains extinct throughout a 360o rotation of the stage, then the mineral or substance on the microscope stage is probably isotropic. |
|
Determination of Refractive Index for Isotropic Solids:The Immersion Method In isotropic substances, there are only two optical properties that can be determined. One of these is the absorption color, as discussed above. The other is the refractive index. Tables of refractive indices for isotropic minerals, list only the refractive index for one wavelength of light. The wavelength chosen is 589 nm, which corresponds to a yellow color. Such a wavelength would be given off of a sodium vapor lamp. Since these are expensive and generate much heat, sodium vapor lamps are not generally used in optical mineralogy. Instead we use white light. Still, as we shall see later, we can determine the refractive index for 589 nm. The determination of the refractive index of an isotropic substance is made by making a comparison with a substance of known refractive index. The comparison materials used are called refractive index oils. These are smelly organic oils that are calibrated over a range of refractive indices from 1.430 to 1.740 at intervals of 0.005. As you will see in lab, grains of the unknown substance are placed on a glass slide, a cover glass is placed over the grains, and a refractive index oil is introduced to completely surround the grains. This is called the immersion method. |
| The grains are then observed with the analyzer not inserted. If the grain has a refractive index that is very much different from the refractive index of the oil, then the grain boundaries will stand out strongly next to the surrounding oil. The grain will then be said to show high relief relative to the oil. High relief indicates that the refractive index of the grain is very much different from the refractive index of the oil. It does not tell us if the refractive index of the grain is less than or greater than the oil. | 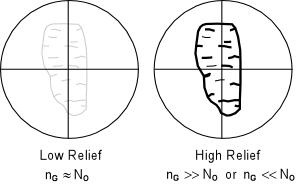 |
| If the refractive index of the grain and the oil are closer, then the
outline of the grain will not stand out as much from the oil. In
this case, the grain is said to low relief
relative to the oil. Again, low relief only indicates that the grain
and oil have similar refractive indices, and does not indicate that
the grain as a lower or higher refractive index than the oil.
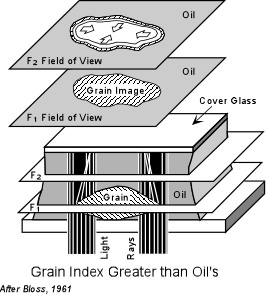
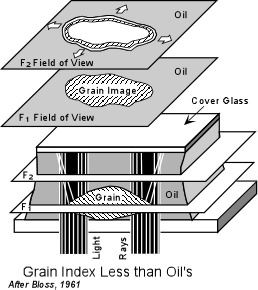
If the refractive index of the grain is exactly the same as the refractive index of the oil, the boundaries of the grain will not be visible. That is to say that the grain will completely disappear in the oil. In this case the grain is said to have no relief relative to the oil. In order to determine whether the grain or the oil has a higher refractive index, a method called the Becke Line Method is used. The Becke Line Method A grain surrounded by oil when viewed through the microscope focused slightly above the position of sharpest focus will display two lines, one dark and one bright that concentric with the border of the grain. The brighter of these lines is called the Becke line and will always occur closest to the substance with a higher refractive index. This can be used to determine if the grain or the oil has the higher refractive index. |
| To use this method, one first focuses the microscope as sharply as
possible on the grain of interest. It is also useful to use the iris
diaphragm to cut down the incoming light as much as possible. This
will make the Becke line stand out better. Then using the fine
focus dial adjustment the microscope stage is lowered (or the objective
lens is raised) slightly out of focus. During this increase in focal
distance one observes a moving bright Becke line. If the Becke line
moves inward, the refractive index of the grain is greater than the
refractive index of the oil.
It is important to remember that the Becke line test is performed by increasing the distance between the grain and the objective lens. Thus, you should not memorize which way to turn the focusing knob, because it may be different with different brands of microscopes. |
 |
| Note also that if the focal distance is decreased, rather than increased, then the opposite results will be obtained, that is with decreasing focal distance the bright Becke line will move into the substance with lower refractive index. |
| For a grain with a refractive index less than that of the oil, the
opposite effect will be observed. When raising the objective lens or
lowering the stage so that the grain goes slightly out of focus, if the
bright Becke line moves into the oil, then the oil has a refractive index
greater than that of the grain.
This method can thus be used to start to narrow down the refractive index of the grain. For example, lets say that we first put grains of an unknown mineral in an oil with a refractive index of 1.540. In this oil, let's say the Becke line test shows that the oil has a higher refractive index than the grain. We could then choose for our next test to put the unknown grains in an oil with a lower refractive index. If the relief is very high, then we would know to choose an oil with a much smaller refractive index. |
 |
| Thus, by performing several tests in several different oils, we could
eventually find an oil that has a refractive index that exactly matches
that of the grain. In such a case the grain would have no relief
relative to the oil and would thus disappear in the oil.
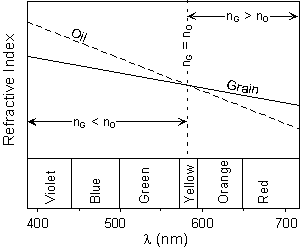
But, this would not necessarily be true of the Becke lines. Recall from above that we said that refractive indices for grains (and also oils) are reported for a specific wavelength of light. That wavelength is 589 nm, which corresponds to yellow. Since we are using white light as an illuminator for our grain, the Becke line will be different for different wavelengths or colors of light. |
| This can be seen by examination of dispersion chart, as shown
here. This is simply a plot of refractive index versus wavelength.
Typical oils used in optical mineralogy generally show a dispersion curve
with a steeper slope than that of minerals. Thus, when we have an
exact match of refractive index between the grain and the oil for yellow
light, we will still see a orange to red colored Becke line that will be
move into the grain, and blue to violet colored Becke line that will move
into the oil.
|
 |
| One other property we can determine for all minerals (including anisotropic minerals) is cleavage or fracture. This can be seen because it is usually necessary to crush or break minerals to obtain a size suitable for mounting in oils. This property can best be seen with the analyzer not inserted. |
| Minerals or glasses that show concoidal fracture will have curved grain boundaries. If a single cleavage direction is present, then it will show as parallel grain boundaries. Sometimes, the cleavages can be seen as breaks within a grain as well, although this is more common in thin sections than in grain mounts. Two cleavage directions will show as intersecting straight sided grain boundaries. Three or more cleavage directions should be visible as well, but it must be remembered that the microscope view is nearly 2-dimensional, so you may be able to see only 2 cleavage directions at once. | 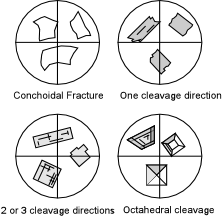 |
Summary of Observations of Isotropic Minerals
Next time we will look at the class of anisotropic minerals called uniaxial minerals. |
|
|