| EENS 2110 |
Mineralogy |
| Tulane University |
Prof. Stephen A. Nelson |
|
Twinning, Polymorphism, Polytypism, Pseudomorphism |
|
|
|
|
Twinning in Crystals Sometimes during the growth of a crystal, or if the crystal is subjected to stress or temperature/pressure conditions different from those under which it originally formed, two or more intergrown crystals are formed in a symmetrical fashion. These symmetrical intergrowths of crystals are called twinned crystals. Twinning is important to recognize, because when it occurs, it is often one of the most diagnostic features enabling identification of the mineral. |
| What happens is that lattice points in one crystal are shared as lattice points in another crystal adding apparent symmetry to the crystal pairs. Twinning, because it adds symmetry, never occurs in relation to the existing symmetry of the crystal. |  |
Symmetry Operations that Define Twinning Because symmetry is added to a crystal by twinning, twining can be defined by the symmetry operations that are involved. These include:
|
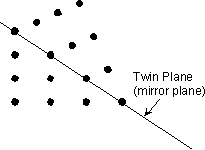
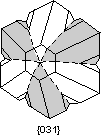
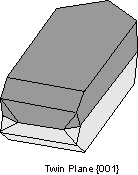
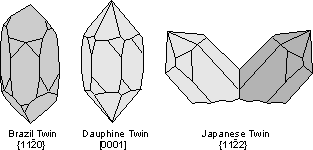
Twin Laws Twin laws are expressed as either form symbols to define twin planes (i.e. {hkl}) or zone symbols to define the direction of twin axes (i.e. [hkl]). The surface along which the lattice points are shared in twinned crystals is called a composition surface.
|
|
If the twin law can be defined by a simple planar composition surface, the twin plane is always parallel to a possible crystal face and never parallel to an existing plane of symmetry (remember that twinning adds symmetry). If the twin law is a rotation axis, the composition surface will be irregular, the twin axis will be perpendicular to a lattice plane, but will never be an even-fold rotation axis of the existing symmetry. For example twinning cannot occur on a new 2 fold axis that is parallel to an existing 4-fold axis. |
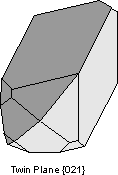
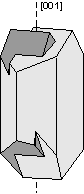
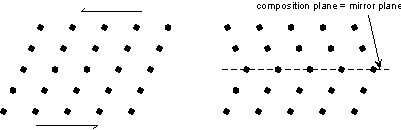
Another way of defining twinning breaks twins into two separate types. |
|
 |
|
 |
|
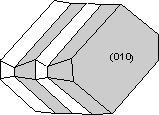
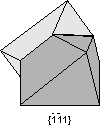
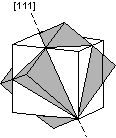
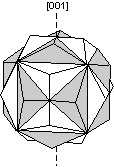
Contact twins can also occur as repeated or multiple twins.
|
 |
|
 |
Twinning can originate in 3 different ways, as growth twins, transformation twins, and glide or deformation twins. |
|
 |
|
|
 |
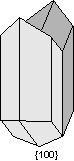
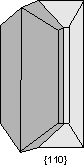
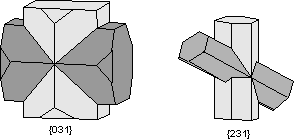
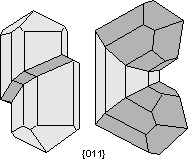
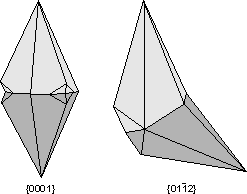
Common Twin Laws
|
|
|
 |
|
|
|
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
|
 |
|
|
 |
|
 |
|
|
|
 |
|
 |
|
|
|
 |
|
|
 |
|
|
 |
|
Polymorphism Polymorphism means "many forms". In mineralogy it means that a single chemical composition can exist with two or more different crystal structures. As we will see when we look more closely at crystal structures, if a crystal is subjected to different pressures and temperatures, the arrangement of atoms depends on the sizes of the atoms, and the sizes change with temperature and pressure. In general, as pressure increases the volume of a crystal will decrease and a point may be reached where a more compact crystal structure is more stable. The crystal structure will then change to that of the more stable structure, and a different mineral will be in existence. Similarly, if the temperature is increased, the atoms on the crystal structure will tend to vibrate more and increase their effective size. In this case, a point may be reached where a less compact crystal structure is more stable. When the crystal structure changes to the more stable structure a different mineral will form. The
change that takes place between crystal structures of the same chemical
compound are called polymorphic transformations. |
|
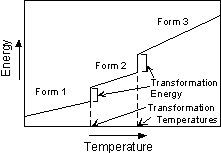
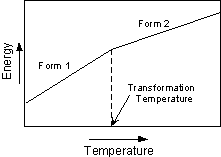
Types of Polymorphic Transformations Stability of crystal structures is generally referred to in terms of the energy of the crystal structure. In general terms this can be thought of as the bond strength (enthalpy), and entropy (degree of order or randomness) of the structure. In general, the structure with the lowest energy is the most stable at any given temperature and pressure. This results in three types of transformations. |
|
 |
|
For example, diamond is a metastable polymorph of Carbon at the pressures and temperatures present at the Earth's surface, yet, as the saying goes "diamonds are forever". Not really, it's just that the rate at which diamond can rearrange its crystal structure to become graphite, the polymorph stable at low P and T, is very slow at the low temperatures found near the Earth's surface. |
|
 |
|
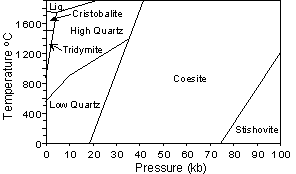
For example, at 1 atmosphere pressure high quartz (b quartz) is the stable form of quartz above 580o C. When high quartz is brought to a temperature below 580o it immediately is transformed into low quartz (a quartz). Thus, high quartz is never seen in rocks at the surface of the Earth. |
|
Important Polymorphs Many common minerals show polymorphism. We here look at some of the more common ones. |
|
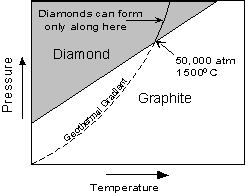 |
|
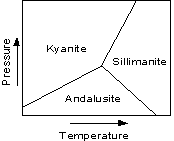 |
|
|
 |
|
Cristobalite and tridymite can exist metastably at the low
temperatures near the Earth's surface, and thus are found in
rocks. But high quartz will also transform to low quartz before it
reaches temperatures present at the Earth's surface, so it is never
found in rocks. |
|
|
Polytypism Polytypism is a type of polymorphism wherein
different polymorphs exist in different domains of the same crystal.
It has to do with the way that individual layers are stacked within a
crystal structure. Polytypism has little geologic consequence, and
will thus not be discussed further here. |
|
Metamict Minerals Metamict minerals are minerals whose crystal structure has been partially destroyed by radiation from contained radioactive elements. The breakdown of the crystal structure results from bombardment of a particles emitted by the decay of U and Th radioactive isotopes. The mineral zircon (ZrSiO4) often has U and Th atoms substituting for Zr in the crystals structure. Since U and Th have radioactive isotopes, Zircon is often seen to occur in various stages of metamictization. |
|
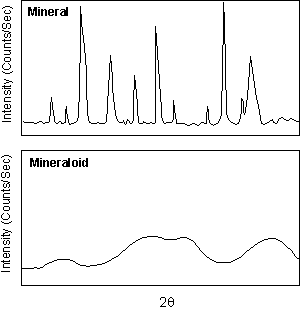
Mineraloids By definition, a mineral has to have an ordered atomic arrangement, or crystalline structure. There are some Earth materials that fit all other parts of the definition of a mineral, yet do not have a crystalline structure. Such compounds are termed amorphous (without form). Some of these amorphous compounds are called mineraloids. These usually form at low temperatures and pressures during the process of chemical weathering and form mammillary, botryoidal, and stalactitic masses with widely varying chemical compositions. Limonite [FeO.(OH).nH2O] and allophane ( a hydrous aluminum silicate) are good examples. |
| Others like volcanic glass and opal (SiO2.nH2O)
have short-range order or domains wherein some crystalline-like order
exists.
Unlike crystalline minerals that show sharp, well defined x-ray diffraction peaks, these mineraloids with short-range order show broad diffraction peaks that give evidence of the short-range order. |
 |
|
Pseudomorphism Pseudomorphism is the existence of a mineral that has the appearance of another mineral. Pseudomorph means false form. Pseudomorphism occurs when a mineral is altered in such a way that its internal structure and chemical composition is changed but its external form is preserved. Three mechanisms of pseudomorphism can be defined:
|
Examples of questions on this material that could be asked on an exam
|