| EENS 2120 |
Petrology |
| Prof. Stephen A. Nelson |
Tulane University |
|
General Classification of Igneous Rocks |
|
Classification of igneous rocks is one of the most confusing aspects of geology. This
is partly due to historical reasons, partly due to the nature of magmas, and partly due to
the various criteria that could potentially be used to classify rocks.
|
Because of the limitations of the various criteria that can used to classify igneous
rocks, geologists use an approach based on the information obtainable at various stages of
examining the rocks.
|
|
Simple Field Classification of Volcanic Rocks |
||
| Rock Name | Essential Minerals* | Other Minerals (may or may not be present) |
| Basalt | Olivine | Cpx, Opx, Plag. |
| Basanite | Olivine + Feldspathoid (Nepheline/ Leucite) | Cpx, Plag. |
| Andesite | No olivine, abundant Plagioclase | Cpx, Opx, Hornblende |
| Trachyte | Sanidine + Plagioclase | Na-Cpx, Hornblende, Biotite |
| Dacite | Plagioclase + Hornblende | Cpx, Opx, Biotite |
| Rhyolite | Quartz | Sanidine, Biotite, Plag., Hornblende, Cpx, Opx |
| * The amount of glass in the groundmass increases, in general, from the top to the bottom of the chart. | ||
|
| Note that at each stage of the process, the classification may change, but it is important to keep in mind that each stage has limitations, and that classification at each stage is for the purposes of describing the rock, not only for the individual investigator, but anyone else. Thus, the classification scheme should be employed in a consistent manner so that later investigators can understand what you are talking about at each stage of the process. |
General Chemical Classifications SiO2 (Silica) Content
This terminology is based on the onetime idea that rocks with a high % SiO2 were precipitated from waters with a high concentration of hyrdosilicic acid H4SiO4. Although we now know this is not true, the acid/base terminology is well entrenched in the literature. Silica Saturation If a magma is oversaturated with respect to Silica then a silica mineral,
such as quartz, cristobalite, tridymite, or coesite, should precipitate from the magma,
and be present in the rock. On the other hand, if a magma is undersaturated with
respect to silica, then a silica mineral should not precipitate from the magma, and thus
should not be present in the rock. The silica saturation concept can thus be used to
divide rocks in silica undersaturated, silica saturated, and silica oversaturated rocks.
The first and last of these terms are most easily seen.
|
Nepheline- NaAlSiO4 Leucite - KAlSi2O6 Forsteritic Olivine - Mg2SiO4 Sodalite - 3NaAlSiO4.NaCl Nosean - 6NaAlSiO4.Na2SO4 HaŁyne - 6NaAlSiO4.(Na2,Ca)SO4 Perovskite - CaTiO3 Melanite - Ca2Fe+3Si3O12 Melilite - (Ca,Na)2(Mg,Fe+2,Al,Si)3O7
Thus, if we find any of these minerals in a rock, with an exception that we'll see in a moment, then we can expect the rock to be silica undersaturated. If we calculate a CIPW Norm (we'll see how to do this in lab) the normative minerals that occur in silica undersaturated rocks are nepheline and/or leucite. To get an idea about what silica saturation means, let's look at a simple silicate system - the system Mg2SiO4 - SiO2 |
| Note how compositions between Fo and En will end their crystallization
with only Fo olivine and enstatite. These are SiO2-undersaturated.
compositions. All compositions between En and SiO2 will end their
crystallization with quartz and enstatite. These are SiO2 - oversaturated
compositions. Note also that this can cause some confusion in volcanic rocks that do not complete their crystallization due to rapid cooling on the surface. Let's imagine first a composition in the silica-undersaturated field. Cooling to anywhere on the liquidus will result in the crystallization of Fo-rich olivine. If this liquid containing olivine is erupted and the rest of the liquid quenches to a glass, then this will produce a rock with phenocrysts of olivine in a glassy groundmass. |
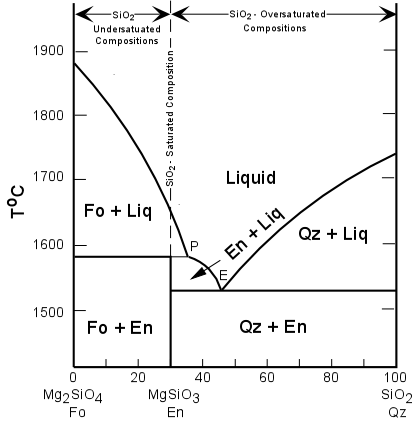 |
| Applying the criteria above for identifying silica undersaturated rocks would tell us that this is a silica-undersaturated rock, which we know to be correct. Next, let's look at a silica oversaturated composition, such as one just to the left of the point labeled 'P' in the diagram. If this liquid is cooled to the liquidus and olivine is allowed to crystallize, and is then quenched on the surface, it will contain phenocrysts of Fo-rich olivine in a glassy groundmass. Applying the criteria above would suggest that this rock is also silica undersaturated, but we know it is not. This illustrates one of the difficulties of applying any criteria of classification to volcanic rocks where incomplete crystallization/reaction has not allowed all minerals to form. |
Alumina (Al2O3) Saturation After silica, alumina is the second most abundant oxide constituent in igneous rocks. Feldspars are, in general, the most abundant minerals that occur in igneous rocks. Thus, the concept of alumina saturation is based on whether or not there is an excess or lack of Al to make up the feldspars. Note that Al2O3 occurs in feldspars in a ratio of 1 Al to 1 Na, 1K, or 1 Ca:
Three possible conditions exist.
|
Alkaline/Subalkaline Rocks One last general classification scheme divides rocks that alkaline from those that are subalkaline. Note that this criteria is based solely on an alkali vs. silica diagram, as shown below. Alkaline rocks should not be confused with peralkaline rocks as discussed above. While most peralkaline rocks are also alkaline, alkaline rocks are not necessarily peralkaline. On the other hand, very alkaline rocks, that is those that plot well above the dividing line in the figure below, are also usually silica undersaturated. |
Examples of Questions on this material that could be on an Exam
|