| EENS 2120 |
Petrology |
| Prof. Stephen A. Nelson |
Tulane University |
|
Metamorphic Mineral Assemblages |
|
The mineral assemblages that occur in metamorphic rocks depend on four factors:
If a rock is taken to some higher pressure and temperature then the mineral assemblage that develops should represent stable chemical equilibrium if the conditions are held for a long enough period of time that equilibrium can be achieved. Since metamorphism usually involves long periods of geologic time, most metamorphic rocks represent an equilibrium mineral assemblage.
The Phase Rule for Metamorphism Recall that the phase rule states that
If you think about it, in metamorphic rocks where temperature and pressure can both vary during metamorphism, the most likely case would be to find a divariant (F=2) assemblage of phases. A univariant assemblage (F=1) would be less likely to occur, and an invariant assemblage (F=0) would represent equilibrium at a fixed point in temperature and pressure, and would thus be even less likely to occur. So, for F=2, C=P, the number of phases present in a rock for the more common divariant assemblage will be equal to the number of components. If P is greater than C, then one of three possibilities exist for the mineral assemblage.
If possibility (1) is the reason for the lack of correspondence with the phase rule, it can usually be determined by close inspection of the rock. Reaction textures present in the rock might indicate incomplete reaction. Known retrograde minerals, i.e. those stable at lower pressures and temperatures than the rest of the minerals in the rock, could be identified. These retrograde phases could then be subtracted from the number of phases being considered and the phase rule could be reapplied to only the phases known to be in equilibrium. (For example, the presence of chlorite in amphibolite and granulite facies rocks would be indicative that the chlorite is a retrograde mineral or mineral produced during weathering, and thus would not be considered in the application of the phase rule.) Possibility 2 could always occur, and if the number of components is
chosen correctly and retrograde minerals are not considered, then this may be the case. The number of components, as stated in the phase rule, must be chosen so as to represent the minimum number necessary to form all phases possible in the rock. Recall that the number of components is not strictly the number of oxide components or the number of elements as reported in a chemical analysis of the rock. If we just consider the major phases that make up metamorphic rocks and consider that some ions freely substitute for one another in solid solutions, then the number of components can often be reduced to 7 or 8. For example:
If H2O and CO2 are assumed to be always present and available to form hydrous and carbonate minerals, then the number of components can be reduced to 5 or 6. Thus for a divariant assemblage (F=2) we would expect to find 5 or 6 different mineral phases present in a metamorphic rock, or up to 8 phases if the assemblage is invariant. This is the basis for the construction of the AKF and ACF diagrams discussed previously, where the number of components have been reduced to 4, by making assumptions like quartz and alkali feldspar can always be present. Still, you are cautioned that the above analysis is not always generally applicable, and each rock must be considered on a case-by case scenario. |
Example of Progressive Metamorphism Progressive or prograde metamorphism
occurs as the temperature and pressure are increased on the rock. As the pressure
and temperature increase, a rock of a given chemical composition is expected to undergo a
continuous series of chemical reactions between its constituent minerals and any fluid
phase present to produce a series of new mineral assemblages that are stable at the higher
pressures and temperatures. We here illustrate how the mineral assemblages might
change in a hypothetical set of rocks, starting with a low grade mineral assemblage as
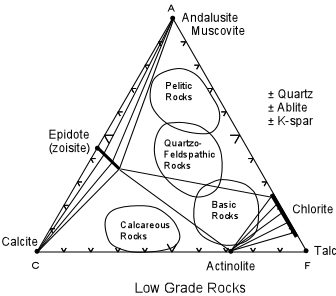
shown in the ACF diagram below. |
| First, consider the compositions of various types of rocks. Pelitic and quartzo-feldspathic rocks are relatively aluminous, calcareous rocks are relatively rich in the C component, and basic rocks are relative rich in the F component. At some low temperature and pressure typical of low grade metamorphism, pelitic and quartzo feldspathic rocks in this example would consist of epidote, chlorite, andalusite, muscovite, and possibly quartz, k-spar, muscovite, and albite. Calcareous rocks would contain epidote, calcite, actinolite, and possibly quartz, albite, and k-spar. Basic rocks would consist of epidote, chlorite, actinolite, and possibly, quartz, albite, and k-spar. Ultramafic rocks would consist of actinolite, chlorite and talc. |
 |
| At some higher pressure and temperature the assemblage might change as follows: pelitic rocks would consist of sillimanite, plagioclase, cordierite, muscovite, and possibly quartz and k-spar (the albite is now included in the plagioclase). Quartzo-feldspathic rocks would consist of plagioclase, cordierite, biotite, and likely quartz and k-spar. Calcareous rocks would consist of grossularite, calcite and hornblende. Basic rocks would contain plagioclase, biotite, hornblende, and perhaps quartz and k-spar. Ultramafic rocks would consist of biotite anthopyllite, and hornblende. |
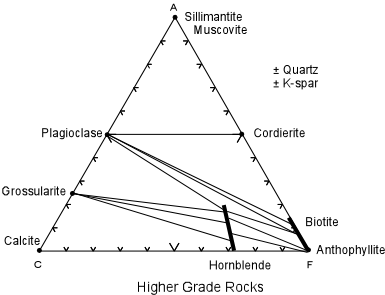 |
| Now let's consider what happened in jumping from the pressure/ temperature conditions
of the first diagram to those of the second diagram. First, we list the phases that
have disappeared and those that have appeared:
|
Phases Disappeared
New Phases
andalusite sillimanite epidote/zoisite plagioclase chlorite grossularite talc cordierite actinolite biotite albite anthophyllite hornblende
| Next, let's try to write the chemical reactions that must have occurred between the
two sets of pressure/temperature conditions that would explain the new mineral
assemblages.
For the disappearance of andalusite and appearance of sillimanite the reaction is simple:
|
Epidote (zoisite) would break down to produce the anorthite component of plagioclase
and grossularite, resulting in the expulsion of water as a fluid phase:
Chlorite could have reacted with muscovite and quartz to produce biotite, cordierite, and fluid:
K(Fe,Mg)3AlSi3O10(OH)2 + (Mg,Fe)2Al4Si5O18
+H2O Talc would break down to produce anthophyllite, quartz, and fluid:
and chlorite, actinolite, epidote (zoisite), and quartz would react to produce hornblende and fluid: 7(Mg,Fe)5Al2Si3O10(OH)2+13Ca2(Mg,Fe)5Si8O22(OH)2+12Ca2Al3Si3O12(OH)+14SiO2
=>
25Ca2(Mg,Fe)4Al2Si7O22 (OH)2
+ H2O |
| With the exception of the first reaction (andalusite => sillimanite) all of these
reactions result in the evolution of H2O in a fluid phase. Such reactions
are called dehydration reactions, and will be discussed further
in the next lecture. Next, let's increase the temperature and pressure so that a new set of minerals develops for each rock. At these new pressure/temperature conditions, pelitic rocks would contain plagioclase, sillimanite, cordierite, quartz, and k-spar. Quartzo-feldspathic rocks would consist of plagioclase, cordierite, almandine/pyrope garnet, quartz, and k-spar. |
| Calcareous rocks would consist either of wollastonite, grossularite and diopside, or grossularite, plagioclase, and diopside, or plagioclase, diopside, and biotite, depending on the composition of the initial rock. Basic rocks would consist of plagioclase, diopside, and almandine/pyrope, or plagioclase, hypersthene, and almandine/pyrope. Ultramafic rocks would consist of hypersthene, almandine/pyrope, and diopside. |
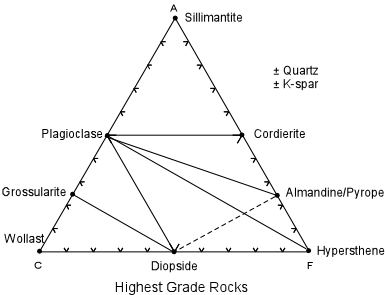 |
| Again, we can make a list of the phases that disappeared and those that appeared at some point between the two pressure/temperature conditions. |
Phases Disappeared
New Phases
muscovite wollastonite calcite diopside hornblende hypersthene anthophyllite almandine/pyrope biotite
| Exploring the reactions that must have taken place to explain the new mineral
assemblage, we proceed as follows:
For the disappearance of muscovite we can write:
For the disappearance of calcite and the appearance of wollastonite we can write:
|
Hornblende would break down to diopside, hypersthene, plagioclase, and fluid.:
Anthophyllite would break down to hypersthene and quartz:
For the breakdown of biotite to form hypersthene and k-spar the following reaction must have occurred:
And finally, for the formation of almandine/pyrope from cordierite and anthophyllite, the following reaction could have occurred:
2(Mg,Fe)3Al2Si3O12 + 3(Mg,Fe)SiO3 +
4SiO2 + H2O |
| Again, all of the reactions evolve a fluid phase. The calcite =>
wollastonite reaction is a decarbonation reaction, and the rest are dehydration reactions.
This illustrates very well the concept we have discussed repeatedly throughout this
course, that prograde metamorphism involves a series of devolatilization reactions that
produce a fluid phase. The fluid phase generally will consist of a mixture of H2O
and CO2. This results in producing minerals that are less volatile rich
with increasing grade of metamorphism. Ultimately, at the highest grade of
metamorphism no hydrous or carbonate bearing phases exist.
Retrograde Metamorphism If retrograde metamorphism were a common process then upon uplift and unroofing metamorphic rocks would progressively return to mineral assemblages stable at lower pressures and temperatures. Yet, high grade metamorphic rocks are common at the surface of the Earth and usually show only minor retrograde minerals. Three factors inhibit retrograde metamorphism, two of which involve the fluid phase. |
Given enough time, all metamorphic rocks will eventually change to an assemblage of minerals stable under conditions present near the surface of the Earth. This process, however, is called weathering, and occurs near the earth's surface.. Next time we will explore in more detail some the factors that govern the chemical reactions that occur during metamorphism. |
Examples of questions on this material that could be asked on an exam
|