| EENS 212 |
Petrology |
| Prof. Stephen A. Nelson |
Tulane University |
|
Metamorphic Reactions, Isograds, and Reaction Mechanisms |
|
Types of Metamorphic Reactions Chemical reactions that take place during metamorphism produce mineral assemblages stable under the new conditions of temperature and pressure. Thus, in order to understand the mineral assemblages and what they mean in terms of the pressure and temperature of metamorphism, we must first explore the various types of metamorphic reactions. A metamorphic reaction is an expression of how the minerals got to their final state, but a reaction does not necessarily tell us the path that was actually taken to arrive at this state. Sometimes it is possible to deduce the path by means of a reaction mechanism. Thus, we will also explore reaction mechanisms. If we are considering a rock of fixed chemical composition, then a metamorphic reaction states the principles of equilibrium. In other words, if we can write a reaction expressing equilibrium between the minerals we see in the rock, we expect that the reaction must have been taking place during metamorphism. We will first look at various types of metamorphic reactions. Univariant Reactions
|
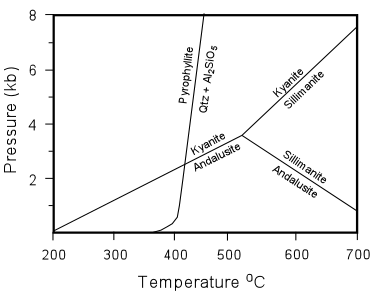
| defines a reaction boundary on a P-T diagram. This boundary can be determined experimentally or can be calculated using thermodynamic properties of the phases involved. If we find a rock that contains pyrophyllite, quartz, and an Al2SiO5 mineral, then we know that metamorphism took place somewhere along the trajectory of the reaction boundary. Furthermore, combining this with the knowledge of the stability fields of the Al2SiO5 minerals, we could place boundaries on the conditions of metamorphism. |
 |
| For example, if the mineral is andalusite, then we know the rock was metamorphosed at
a pressure less than about 2.5 kilobars. If the mineral is kyanite, then we know
that the pressure was greater than about 2.5 kilobars.
Combinations of other such reactions could further constrain the pressure and temperature conditions of metamorphism. The example above, however, is probably too simple for a real rock. |
| Although simple, we can use the diagram to illustrate another point. Imagine that a group of rocks are buried along the geothermal gradient shown in the diagram to the right. Rocks buried to a pressure less than about 4 kb and a temperature less than about 420 oC should have pyrophyllite so long as they have the right composition. Rocks buried to pressures between about 4 and 5 kb and temperatures between 420 and about 600 oC should have kyanite + quartz, and rocks buried to pressures along the geothermal gradient greater than about 5 kb and temperatures greater than about 600 oC should have Sillimanite + Quartz. | 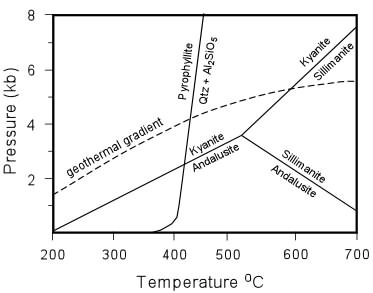 |
| Now imagine that these rocks are brought back to the surface of the Earth and that retrograde metamorphism did not occur on the reverse path back to the surface. Furthermore, they are fortuitously exposed so that the strike direction of the rocks is coincident with the direction along which temperature and pressure increased along the geothermal gradient during the metamorphic event. |
| If we walk along the outcrop along the strike direction (coincident with the direction that pressure and temperature increased during metamorphism) we see that in pelitic rocks the low P & T end of the outcrop has a mineral assemblage consisting of only pyrophyllite. Walking further along strike, we suddenly come to a place where the mineral assemblage changes to Kyanite + Quartz. Note that if were making a geologic map, we could draw a line on the map that separates the pelitic rocks containing only Pyrophyllite from those containing Kyanite + Quartz. Such a line (a surface in 3 dimensions) is called an isograd (iso - same, grad - grade). | 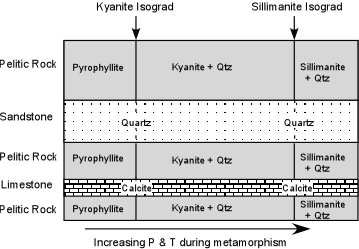 |
| In this case, since it represents the first appearance of Kyanite, we
call it the Kyanite Isograd.
Notice that the isograd represents the point on the phase diagram, above where the geothermal gradient intersects the boundary for the reaction: Al2Si4O10(OH)2 <=> Al2SiO5
+ 3SiO2 + H2O Also notice that if were were walking along an outcrop of the sandstone or the limestone, that we would not be able to map this isograd in these rocks. The reason of course is that the sandstone, made of pure grains of quartz, and the limestone, made of pure grains of calcite, do not have the necessary chemical constituents to form minerals like Pyrophyllite and Kyanite. If we continue walking along the direction that T & P increased in these rocks during metamorphism, we would eventually come to another place where in the pelitic rocks the mineral assemblage changes. This time the change is from Kyanite + Quartz to Sillimanite + Quartz. Again, we can draw a line on the map that indicates this change in mineral assemblage, this time calling it the Sillimanite Isograd. Just like before, this represents the point on the phase diagram, above, where the geothermal gradient intersected the boundary for the reaction: Al2SiO5 <=> Al2SiO5 Because the sandstone and the limestone do not contain Kyanite, the isograd does not appear in these rocks, but we can still extrapolate its position across the map or outcrop. |
| On mineral compatibility diagrams, univariant reactions may show up as flipping tie lines. To illustrate this we will use the AFM diagram for metapelites. |
|
|
| In the diagrams, the average pelitic rock is shown as composition
x. At low grade, the stable mineral assemblage is Garnet + Chlorite
+ Biotite + Muscovite + Quartz. As temperature and pressure are
raised, this assemblage becomes unstable and the tie line connecting
Garnet and Chlorite is replaced by one connecting Staurolite +
Biotite.
The reaction that occurred is the univariant reaction: Garnet + Chlorite + Muscovite = Staurolite + Biotite + Quartz + H2O |
| Imagine now that we are looking at a suite of metamorphic rocks in the field that include a pelitic rocks of composition x. |
| As we follow an outcrop of this rock we note that it contains the mineral assemblage Garnet + Chlorite + Muscovite + Quartz. We eventually reach a point in the outcrop where the mineral assemblage abruptly changes. Chlorite is no longer present, but instead we find Staurolite. We can now place a line on our map that represents the first appearance of staurolite in these rocks and that point represents a point on what we might call the Staurolite isograd. We would then know that the pressure and temperature conditions must have reached the reaction boundary where the reaction listed above took place. | 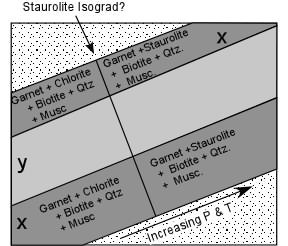 |
| This isograd, however, would probably not be a very good isograd to map, because it would only apply to rocks with a composition similar to composition x. |
| Note that a rock with a composition slightly above x and above the Garnet - Chlorite tie line, like composition y in the AFM diagrams above, would still have a different mineral assemblage above the isograd, but it would be one that contained Staurolite below the isograd, but Biotite above the isograd. So for these rocks we would probably call the isograd the Biotite isograd. Because it would be called the Staurolite isograd for one group of rocks, but the Biotite isograd for another, and because Staurolite would occur in some rocks below the isograd and in other rocks only above the isograd, it would soon become very confusing. | 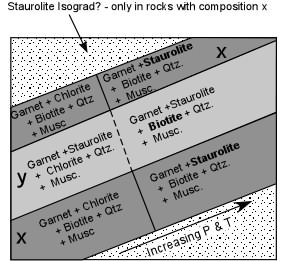 |
| For these rocks the reaction boundary would really represent the disappearance of Chlorite. But since isograds are generally named for the appearance of a mineral with increasing grade, we can't call the reaction boundary the Chlorite Isograd because Chlorite is found in rocks of decreasing grade. |
| Another type of univariant reaction, however represents a reaction that is terminal to a mineral phase for a wide variety of compositions. Let's consider what happens in these same pelitic rocks if we reach a temperature and pressure region where staurolite becomes unstable. |
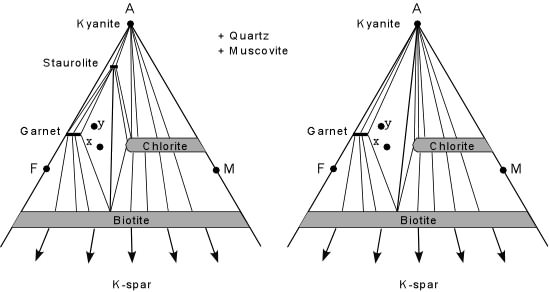 |
| In this case, with increasing temperature, Staurolite becomes unstable
and reacts to produce Garnet, Biotite and Kyanite. Notice that both
rock compositions x and y have the same mineral assemblages before and
after the reaction, although the proportions of the different minerals
will be different in the two rocks. The reaction that is terminal to
Staurolite can be written as:
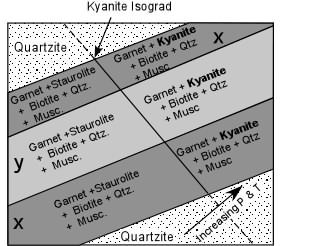
Staurolite + Quartz + Muscovite <=> Garnet + Biotite + Kyanite + H2O Note that for this reaction the disappearance of staurolite also coincides with the appearance of Kyanite in rocks of composition x and y. If we were to encounter this reaction in the field, it could be used to define the Kyanite Isograd. |
|
Note that the Kyanite Isograd as shown here would not be seen in a non-pelitic rock such as the quartzite, but could be extrapolated across the quartzite to other outcrops of pelitic rocks. Note also, that in this diagram, the direction of increasing pressure and temperature during metamorphism was not parallel to the strike of the original rocks, just as a reminder that direction of increasing grade does not need to coincide with any particular orientation of the rocks. |
 |
| Divariant Reactions In the cases discussed above, the univariant reactions that were considered involved reaching a point in pressure temperature space where a reaction occurred resulting in a sudden change in mineral assemblage. These reactions can be considered discontinuous reactions because they occur along specific pressure temperature curves. Because many minerals are solid solutions, it is also possible to have discontinuous reactions that result in a gradual change in composition of the minerals, but not necessarily the formation of new minerals. These reactions are also considered divariant reactions because they occur over a wide range of pressure and temperature conditions. Consider the hypothetical case of rocks that contain minerals like chlorite and garnet, which are both Mg-Fe solid solutions. The reaction that occurs with increasing temperature (at constant pressure) is: Chlorite + Qtz => Garnet + Mg-richer Chlorite + H2O |
| This reaction begins at a temperature of T1 where an initial Mg-poor garnet is produced. As temperature increases, say to T2, both the garnet and the chlorite become more Mg-rich. The reaction continues over a range of temperature until eventually the temperature reaches T3 at which point the much more Mg-rich chlorite disappears leaving garnet with Mg/(Mg/Fe) ratio the same as that in the initial chlorite. We say that this reaction is a continuous reaction because there is no change in mineral assemblage between T1 and T3, but there is a reaction occurring and its effect is to change the compositions of the solid solution minerals. Note the similarity of this idea to the melting behavior of Fe-Mg solid solution minerals, and the similarity to the concept of the continuous reaction series discussed with regard to Bowen's reaction series for magmas (where it involved changes in plagioclase composition). | 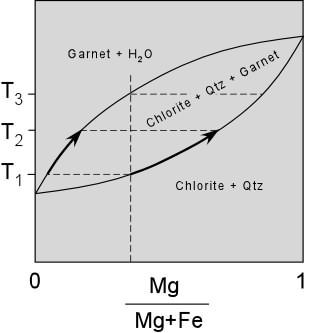 |
| In a more realistic situation we can see how this occurs in relation to a similar reaction on an AFM diagram. |
|
|
| In the diagram to the left, representing the lowest grade, a rock with
composition x consists of a relatively Mg-poor biotite + Mg-poor
chlorite. As metamorphic grade increases to the middle diagram, the
three phase triangle - Garnet - Biotite - Chlorite has shifted, with all
three phases becoming more Mg-rich, and now composition x lies inside this
3 phase triangle, and thus now contains garnet. Further increase in
metamorphic grade, causes further shift to of the apices of the 3 phase
triangle toward more Mg-rich compositions and eventual disappearance of
chlorite from the rock with composition x.
Note that throughout the changes in P & T, the proportion of the 3 phases in composition x will change, as will the composition of the Fe-Mg solid solution minerals. Although the appearance of Garnet will be sudden, the disappearance of chlorite will be gradual as P and T change. The reaction that is taking place in this case is: Muscovite + Chlorite + Quartz <=> Mg-richer chlorite + Biotite +Garnet + H2O |
Metamorphic Reaction Mechanisms As we discussed previously, the fluid phase plays an important role in metamorphic reactions. Not only are fluid components necessary to form any hydrous or carbonate minerals, but the fluid phase can act as a catalyst for metamorphic reactions. We also noted above that the chemical reactions we write for the various transformations of mineral assemblages with increasing pressure and temperature are the net result of what actually took place. For example, we may deduce from a series of metamorphosed pelitic rocks that kyanite reacted to form sillimanite and the reaction: Al2SiO5 => Al2SiO5 must have occurred at some point during metamorphism. But, although this is the net reaction that must have occurred, we don't really know if the andalusite reacted directly to produce the sillimanite or if the andalusite was first dissolved in a fluid phase, with the fluid phase carrying the dissolved components to a new location where the fluid then precipitated the sillimanite. In general, solid-solid reactions run rather slowly because ions have to diffuse through the solids in order to rearrange themselves into new phases. Thus, it seems likely that the actual mechanisms or paths that a reaction takes will be more complicated than the net reaction implies. |
|
If we can gain some insight into reaction mechanisms, then we may be better able to envision exactly how metamorphism operates in the Earth. Metamorphic petrologists can only rarely look through a rock and determine what actually happened. |
| One case where this seems to be possible is in series of metapelites in Canada. D.M. Carmichael examined thin sections of one particular metapelite that contains both kyanite and sillimanite. The rock shows no evidence that the sillimanite was forming directly from kyanite. Instead, examination of the rock in thin section shows that the kyanite has rounded edges and corners that could have formed as a result of dissolution. Similarly, some crystals of quartz in the rock show evidence dissolution. The sillimanite is found growing within flakes of muscovite. Thus, Carmichael deduced that in the area around where the kyanite crystals were dissolving the following reaction was taking place: |
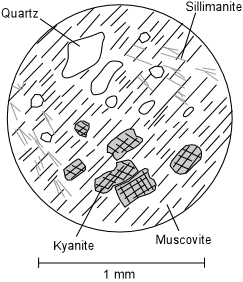 |
|
|
Note that if these two reactions are added together algebraically, the net reaction is still: Al2SiO5 => Al2SiO5 |
| This makes sense, because Al is not very soluble in water, whereas K is soluble. Thus the fluid will be able to easily transport the K ions over short distances to supply that necessary to precipitate muscovite, and the growing sillimanite can use the Al left behind by the fluid. This reaction mechanism is shown diagrammatically in the diagram to the right. It implies that the two reactions were taking place simultaneously in different domains within the rock, and that the fluid phase was important in transferring ions from one domain to another, although over small distances. | 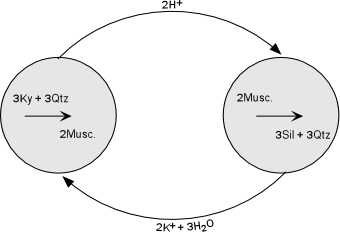 |
|
Estimating Pressure and Temperature of Metamorphism Using combinations of reactions that have likely taken place during metamorphism, petrologists have been able over the years to determine the pressure and temperature of metamorphism in a variety of rocks, and in so doing have been able to place constraints on the fields of temperature and pressure for the various metamorphic facies. Some of these reactions are shown in the diagram below with reaction boundaries superimposed over the facies diagram. |
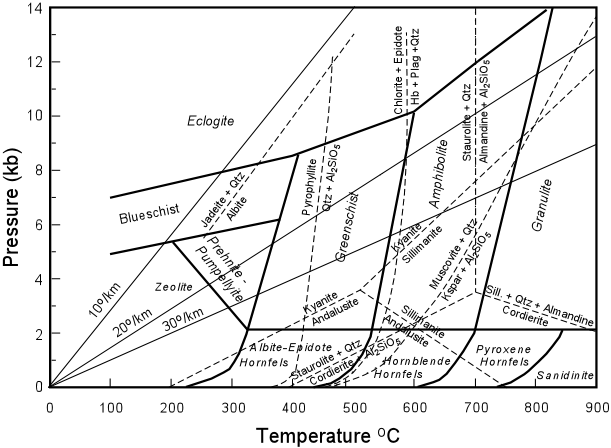 |
The diagram also shows various geothermal gradients that would control the succession
of facies encountered during prograde metamorphism if the rocks were pushed down into the
Earth along one of these geothermal gradients.
|
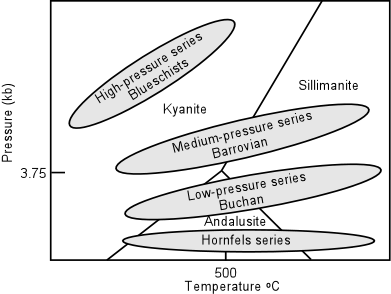 |
Note that a slightly higher geothermal gradient would produce the same succession of
facies, but pelitic rocks would show a change in the Al2SiO5
minerals from kyanite to andalusite to sillimanite. This facies series is
called the Low-pressure series or Buchan facies series. |
Examples of questions on this material that could be asked on an exam
|