| In our discussion of ternary phase diagrams, we will first review and
then expanding on
material covered last semester in Earth Materials on binary and ternary
phase diagrams. You should therefore spend some time reviewing this
material. Links are as follows -
http://www.tulane.edu/~sanelson/eens211/2compphasdiag.html or for
the PDF version -
http://www.tulane.edu/~sanelson/eens211/2compphasdiag.pdf
and
http://www.tulane.edu/~sanelson/eens211/ternaryphdiag.htm or for the PDF
version -
http://www.tulane.edu/~sanelson/eens211/ternaryphdiag.pdf
Bring both sets of lecture notes with you to class. After reviewing
this material we will proceed with the following discussion:
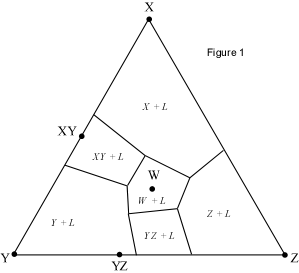
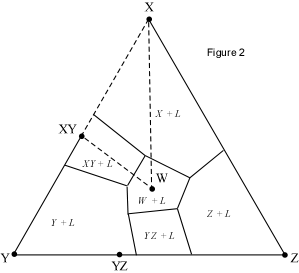
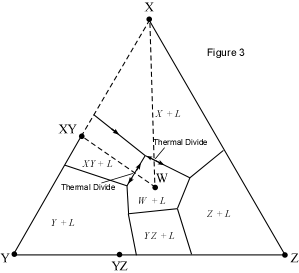
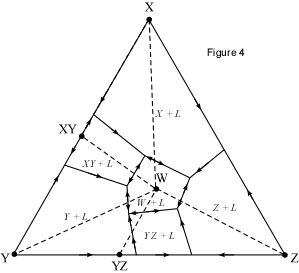
When presented with a complex phase diagram, the first thing one must do
is understand what phases can coexist during crystallization or melting, and
what phases coexist in all possible subsolidus assemblages. Remember that in
a ternary system at constant pressure, the maximum number of phases that can
coexist is 4, and 4 phases can only exist at ternary invariant points (F=
C+1-P, if F=0, C=3, then P = 4). Knowing what phases must be present in the
subsolidus assemblage for any composition in the system is important,
because it tells us where the crystallization path will lead, i.e. to which
of the invariant points in the system where 3 solid phases and a liquid will
coexist prior to the disappearance of the liquid phase. Univariant curves,
also called cotectics, are lines along which 3 phases coexist at constant
pressure (F =1, C=3, so P = 3). Such curves bound the primary phase fields
(divariant fields), and by inspection of the phases that exist on either
side of the curves, one can tell which phases are crystallizing as the
liquid composition moves along the curve. Furthermore, in the absence of
isotherms or other temperature information, one can determine the
down-temperature direction that a liquid will move along such a curve. The
method is as follows:
|