
Ph.D. (LSU, 1988)
1430 Tulane Ave., Box SL-43
New Orleans LA 70112
 |
Ph.D. (LSU, 1988) |
| Phone: (504) 988-3990 | |
| FAX: (504) 988-2739 | |
| Address: 1430 Tulane Ave., Box SL-43 New Orleans LA 70112 |
|
| Email: landry@tulane.edu |
Our work employs biochemical and biophysical techniques including
nuclear magnetic resonance (NMR) spectroscopy to investigate
structure/function
relationships in protein-protein interactions, especially those
involved
in cellular stress and immune responses.
Structural Instability and T-cell Epitope Immunodominance.
Humoral and cellular immunity to HIV and other pathogens depend on
antigen-specific
CD4+ T cell responses directed against peptides presented in MHC class
II antigen presenting proteins. Generally, CD4+ T-cell responses are
focused
on only a few immunodominant regions of naturally processed antigens.
We
have found that immunodominant regions occur in the sequences flanking
locally unstable segments of an antigen's three-dimensional structure,
and we are now modifying CD4+ helper T-cell immune responses to
recombinant
antigens by engineering their processing and presentation.
E. coli DnaJ/DnaK. Members of the Hsp40 and Hsp70
families
of chaperones are implicated in a host of cellular processes in all
organisms
including DNA replication, gene expression, and protein translocation
across
membranes, in addition to the stress response. The goal of this
research
is to understand at a physical chemical level how Escherichia coli
molecular chaperones DnaJ (an Hsp40) and DnaK (an Hsp70) cooperate in
the
management of protein-protein interactions.
Mechanism of Hsp60/Hsp10 Chaperonin Specificity. Chaperonins are a class of stress-induced molecular chaperones characterized by a ring-shaped oligomeric structure and the distinctive ability to use ATP to promote protein folding. Our studies aim to illuminate mechanistic features of chaperonin function and identify determinants of Hsp60 specificity for substrates and Hsp10s. [Link to "More on GroES"].
In our work on T-cell epitope immunodominance, we investigate how the immune system zooms in on key features of foreign and self proteins, as it fights off invaders but avoids causing autoimmune disease. The immune system recognizes bacteria, viruses, and toxins through surveillance by antibodies and T-cell receptors. Antibodies act alone to recognize intact foreign antigen molecules; whereas, T-cell receptors recognize pieces (epitopes) of antigen molecules that have been broken down and presented on the surface of specialized antigen-presenting cells. This dual-aspect recognition nearly ensures that the immune system will not attack our own molecules. Our work has shown that the process of antigen breakdown tends to focus the immune system on certain pieces of proteins. We suspect that bacteria and viruses have evolved to misdirect the immune system toward highly variable or otherwise less effective protein segments. We are attempting to overcome this misdirection by engineering subunit vaccines so that the immune system focuses on the most important antigen segments. Understanding how the immune systems distinguishes self from non-self will allow us to develop strategies to treat and prevent asthma and other forms of allergy.
Heat shock protein structure/functionAn important aspect of our approach to understanding biological processes is to analyze the structure and dynamics of proteins. In this work, we utilize biophysical techniques such as nuclear magnetic resonance (NMR) spectroscopy as well as recombinant DNA and other techniques of biotechnology. Proteins are chains of amino acids. With 20 types of amino acids and hundreds of amino acids in a single chain, the "primary" structure of proteins is already complicated; but the chains "fold" into even more complex three-dimensional structures containing various repeating and non-repeating elements, and portions of order and disorder. We have used NMR to determine the structure of heat shock proteins, identify their "active sites", and delineate disordered regions. Recently, we've begun to describe the structural fluctuations in the disordered regions with the goal of understanding how these fluctuations affect the binding of heat shock proteins to each other. Eventually, these studies will show how heat shock proteins promote the proper folding and assembly of other proteins. Aside from their role in the stress response, heat shock proteins also have "housekeeping" functions in which they act as "molecular chaperones" to keep proteins from engaging in unproductive interactions. Some evidence suggests that heat shock proteins are involved in the development and propagation of amyloid diseases such as Alzheimer's disease and Mad Cow disease, in which certain proteins misfold into a toxic form.
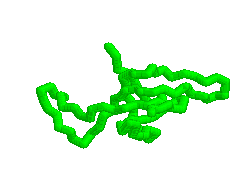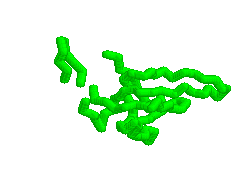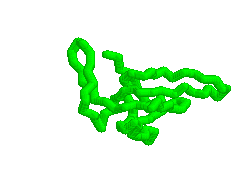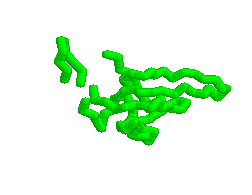
Animation of various conformations of the GroES mobile loop.
Current
Former
List of publications.
| Tulane Biochemistry Home |