AIDS, or acquired immunodeficiency syndrome, has had a major impact on several diseases caused by protozoan pathogens. The etiological agent of AIDS is a retrovirus called human immunodeficiency virus (HIV). Retrovirues are enveloped, single-stranded RNA viruses. Following the infection of a cell, a virus-encoded enzyme--called reverse transcriptase or RNA-dependent DNA polymerase--copies the RNA genome into DNA which is integrated into the genome of the host cell. Copies of the viral genome and mRNA are transcribed from the integrated retrovirus and the resulting viral proteins are assembled with the RNA genome into viral particals which bud from the host cell.
The cellular receptor for HIV is the CD4-antigen and a co-receptor from the b-chemokine family (eg., CXCR4, CCR5). All T-helper cells express CD4 on their surface as well as many macrophages and monocytes and some B-lymphocytes. The most overt pathological effect of HIV infection is the reduction in number and the effectiveness of CD4+ T-lymphocytes. Diagnosis of AIDS is defined as being HIV-antibody positive and having CD4+ T-lymphocyte counts below 200 per mm3 or less than 14% of the total lymphocytes. These effects on the various immune effector cells, and especially the CD4+ T-lymphocytes, lead to a general failure of cell-mediated immune responses, and thus, give rise to a range of opportunistic infections. This opportunism can be exemplified as:
| Pieniazek
et al (1999) Emerg. Inf. Dis. 5:444 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
*Genotypes 1 and 2 previously defined (see Cryptosporidium). |
The immune system effectively eliminates or controls most potentially infectious organisms. AIDS and other immuno-compromising conditions will affect the balance between pathogen and host and allow organisms to replicate to higher than normal levels, thus producing more severe disease. The immunosuppression associated with AIDS also allows some organisms, which would normally be unable to effectively establish an infection in humans, to produce disease. For example, in one study the small subunit rRNA was sequenced from the Cryptosporidium of 10 randomly selected HIV patients. Nearly half of the genotypes were from Cryptosporidium normally associated with pets (Table, Pieniazek).
 |
| Modified from Gradoni and Gramiccia (1994) Parasitol. Today 10:264. |
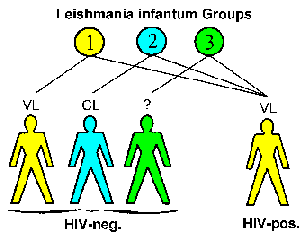
Clinical manifestations may also be affected by AIDS. For example, three different etiological groups, that can also be distinguished by zymodemes, of Leishmania infantum have been described: group 1 usually elicits a visceral disease, group 2 a cutaneous lesion, and group 3 does not cause disease. In HIV-infected persons, all three groups cause a viseral disease (Figure) indicating that normally dermotropic strains can become viscerotropic.
There are either contradictory results or limited apparent affects of co-HIV infection associated with Plasmodium, Entamoeba histolytica, Giardia, Trichomonas vaginalis and Trypanosoma infections. This lack of clear associations could be explained in part by small differences between HIV-negative and HIV-positive indivuals, as opposed to the overt differences observed with the opportunistic infections discussed above. For example, the results of a Ugandan study involving 484 participants making 7220 clinic visits between 1990 and 1998 did show an increase frequency of clinical malaria and parasitemia associated with HIV-1 infection (Table, Whitworth). Futhermore, lower CD4-cell counts were associated with increased parasite densities and increased risk of clinical malaria. These results could have important public health implications when one considers the prevalence of both HIV and malaria in sub-Saharan Africa.| Whitworth
et al (2000) Lancet 356:1051 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Epidemiology studies also suggest that malaria is a deadly co-factor for AIDS. For example:
Interestingly, in vitro studies indicate that CD4-cells stimulated with malarial antigens exhibit a 30-100-fold increase in viral load.
 |
 |
 |
 |
|
Pneumocystis as a Fungus |
|---|
Cyst Wall
|
Pneumocystis was described almost simultaneously by Chagas in 1909 and Carini in 1910. It was first recognized as a human pathogen in association with institutional (i.e., orphanages) outbreaks among malnorished infants and children during and after the second world war. In the recent past and currently it is a frequent and serious opportunistic infection associated with AIDS. Before the widespread use of prophylaxis, PCP was the AIDS-defining illness in 60% of the cases and eventually affected 80% of AIDS patients.
Historically Pneumocystis has been of an uncertain taxonomic status as to whether it is a protozoan or fungus. Molecular data indicates that it is probably an ascomycete-like fungi with no extremely close relatives (Stringer, 1993; J. Inf. Dis. 2:109). Similar conclusions had been reached previously based on ultrastructural studies (Varva and Kucera, 1970; J. Protozol. 17:463). DNA analaysis indicates that Pneumocystis isolated from different host species have very different DNA sequences. In recognition of this genetic distinctiveness it has been suggested to rename Pneumocystis isolated from humans P. jirovecii (Stringer et al, 2002, Emerg. Inf. Dis. 8:891).
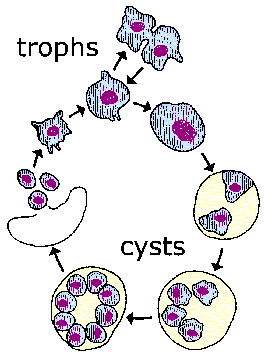
The life cycle of Pneumocystis is not known. Three morphological forms (Figure) have been described in the pulmonary alveoli: trophozoite, cyst, and intracystic bodies. The trophozoite is 2-6 µm in diameter and has pseudopodia. This trophic form presumable replicates by binary fission and is capable of developing into the cyst. The cyst has a thick wall which stains with methenamine-silver and is 4-7 µm in diameter. Up to 8 intracystic bodies (1-1.5 µm) are found within the cyst. These 'zoites' are released following rupture of the cyst wall and presumably develop into trophozoites. Sexual replication has been postulated, but it is not clear when this occurs.
The mode of transmission is not known but presumed to be via inhalation of cysts (or other infective stage). Airborne transmission has been demonstrated in rats and is the most likely mechanism for human transmission. Reactivation of latent infections has long been considered the primary explanation for PCP in immuno-compromised individuals. However, more recent data provide strong circumstantial evidence for an active acquisition of the infection (Beard et al, 2000; Emerg. Inf. Dis. 6:265). These observations include:
Furthermore, high seropositive rates in normal populations, and the rapid rates at which infants become infected and AIDS patients become reinfected after successful treatment suggests that Pneumocystis is a commonly encountered organism. At this time it is not clear if the source is direct (i.e., person-to-person) or indirect (i.e., environmental). One study (Medrano et al, 2005; Emerg. Inf. Dis. 6:245) suggested that humans may be a reservoir for the transmission of the infection. In regards to an indirect transmission, one can postulated a dimorphic fungus (i.e., hyphae vs. yeast forms) which has not yet been identified or characterized from environmental sources. The major risk factor for acquiring the disease is a defective cell-mediated immunity (eg., CD4+ T-lymphocyte counts <200/mm3).
 |
 |
 |
 |
|
Low power magnification of lung section showing alveoli filled with foamy vacuolated material and infiltration of the alveolar septa. |
Pneumocystis is an extracellular pathogen that produces an interstitial plasma cell pneumonia (inflamation of the lungs). The 'interstitial' refers to a thicking of the aveolar septa due to the infiltration of plasma cells (i.e., lymphocytes), which can progress to a fibrosous. In addition, the alveoli fill with a foamy vacuolated material (Figure). The alveoli continue to fill with this material as the disease progresses and death is due to a progressive aphyxia (i.e., suffocation).
The clinical symptoms of PCP are:
Dyspnea, non-productive cough and fever represent a classic triad of symptoms observed in approximately 50% of the cases with nearly all of the patients having two of these symptoms. Symptoms tend to progress slowly over weeks to months and a longstanding, progressive exertional dyspnea is often noted. However, the disease can have a more rapid onset occuring over a period of days in non-AIDS immunosuppressed children and adults. Non-AIDS patients tend to be premature malnourished infants, although it may occur in normal infants without evidence of immune deficiency. Again, the onset may be subtle and occur over several weeks or months characterized by a failure to thrive leading to a rapid respiratory rate (up to 100 respirations per minute) and finally cyanosis. Extrapulmonary (eg., heart, spleen, skin, eyes) Pneumocysistis is rare, but being recognized more frequently. Presumably, dissemination involves a breakdown of the endothelial barriers in the lung.
 |
 |
 |
 |
|
PCP Diagnosis |
||||||||
|---|---|---|---|---|---|---|---|---|
|
||||||||
|
||||||||
|
Gomori's methenamine silver stain is the preferred method for aspirates and biopsies. The cyst wall stains a dark gray to black and is easily recognized (Figure). Preparations stained with Giemsa appear as open areas with up to eight dots (the nuclei of the intracystic bodies). Giemsa only stains the organism and not the cyst wall, whereas only the cyst wall is stain with silver. In addition, single free dots, probably the nuclei of the trophozoites, can also be found throughout the Giemsa-stained specimen (Figure). Diagnosis of Giemsa-stained preparations is difficult and requires a greater level of technical expertise. (See The Art of Cytology by Suzanne L. Adams for a composite color pencil illustration of the various cytological features of Pneumocystis, or Webpath for additional pathology pictures.)
The first choice of treatment trimethoprim-sulfamethoxazole (Bactrim) given either intravenously or orally. AIDS patients generally require longer treatment, show a persistence of organims, and have more adverse drug reactions. Treatment failures occur 10-20% of the time and adverse drug effects, such as nausea, vomiting, rashes, and bone marrow suppression, frequently occur. Alternative drugs include: pentamidine (intravenous or aerosol), trimetrexate (Neutrexin) + folinic acid (Leucovorin), trimethoprim + dapsone, clindamycin (Cleocin) + primquine, or Atovaquone (Mepron). Co-administration of oxygen is recommended in severe PCP cases. In addition, corticosteroids reduce the risk of respiratory failure and death and moderate to severe PCP. The prognosis for patients developing respiratory failure is poor.
Initiating prophalaxis with Batrim for AIDS patients with <200/mm3 CD4+ T-lymphocytes has become the accepted practice. This approach reduces the development of PCP and prolongs life. Bactrim prophalaxis also reduces AIDS-associated toxoplasmic encephalitis. Dapsone and aerosolized pentamidine (NubuPent) are alternatives to Bactrim in the event of adverse reactions.
For a more detailed review on PCP diagnosis and treatment see:
Wilkin A and Feinberg J (1999) Pneumocystis carinii pneumonia: a clinical review. Am. Fam. Phys. 60:1699-1714.
 |
 |
 |
 |
Microsporidia are obligate intracellular parasites found in a wide range of hosts, but most numerous in insects and fish. They were first described by Louis Pasteur in 1865 when he identifed the spores of Nosema bombycis as the causative agent of pébrine disease (aka, Flechenkrankheit) in the commercially important silkworm. More recently, Nosema apis epidemics have caused massive losses of honey bees throughout the world. The economic importance of bees as pollinators is estimated at 10 billion dollars annually.
The microsporidia exhibit many unique features and traditionally have been considered protozoa and given phylum status (Microspora). However, molecular data indicate that they are more closely related to the fungi. This data includes: an insert in elongation factor-1alpha which is unique to fungi; separate thymidylate synthetase (TS) and dihydrofolate reductase (DHFR) proteins; alpha- and beta-tubulin sequences; and the mitochondrial HSP70 sequence. Also notable is the prokaryote-like ribosomes and rRNA sequences which suggest an ancient lineage. The microsporidia are probably a highly derived fungi with a very small genome (2.5 Mb) due to substantail genetic and functional losses as comparted to other eukaryotic micro-organisms. However, it has been suggested based on the sequence of RNA polymerase II that the microsporidia are a sister group to the fungi (Liu et al, BMC Evol Biol 6:74, 2006).
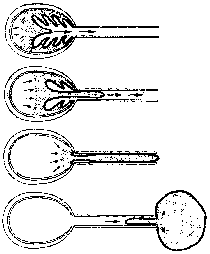
The primary distinguishing feature of microsporidia are a small (1-3 µm) thick-walled spore containing a specialized organelle called the polar tube or polar filament (Figures). The spore wall is composed of a thick protein-rich exospore and a chitinous electron-lucent endospore. The polar filament is a long coiled structure within the spore which is only detectable by the electron microscope. Spore germination involves the rapid extrusion of the polar filament from the anterior end of the spore. The discharged polar tube can range in length from 50-500 µm.
The organism, called a sporoplasm at this stage, is force down the length of this tubule and exits the spore. A structure composed of lamellar membranes, called the polaroplast, is believed to function in the extrusion of the polar filament and the formation of the plasma membrane of the emerging sporoplasm. In some species the polar filament is capable of penetrating the plasma membrane of host cells allowing the microcrosporidia to pass directly into the cytoplasm of the host cell. Alternatively, the spores can be taken up by phagocytosis and the polar tube is used to penetrate the membrane of the phagolysosome and then the sporoplasm gains access to the cytoplasm of the host cell.
|
|
|
 |
||
|
(Left) Electron micrograph of microsporidian spore showing cross-sections of the polar filament (PF). Other denoted structures are: anchoring disk (AD), polaroplast (P), exospore (Ex), endospore (En), nucleus (N), and posterior vacuole (Pv). From T.G. Andreadis (J. Euk. Microbiol. 41:147, 1994) (Center) Schematic representation of spore showing sporoplasm (SP), nucleus (N), polaroplast (P), polar filament (PF), posterior vacuole (Pv) and spore wall (SW). (Right) Schematic representation of of spore germination how polaroplast forms plasma membrane of the emerging sporoplasm and the possible role of osmotic pressure. |
||||
 |
|
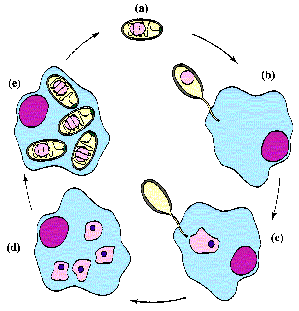
Generalized microsporidian life cycle showing infective phase (a-c), proliferative phase (d), and sporogonic phase (e). Adapted from Keeling and McFadden (Trends Microbiol. 6:19, 1998). |
The microsporidian life cycle comprises three distinct phases: infective, proliferative, and sporogonic (Figure). Spores are the infective forms and are generally acquired by ingestion or inhalation from the environment. Germination results in polar filament extrusion and inoculation of the sporoplasm into the host cell as discussed above. Alternatively, the sporoplasm can be extruded extracellularly and gain access to the host cell by unknown mechanisms. Spores can also be phagocytosed by macrophages and extrusion of the polar filament will allow the oranism to escape from the phagolysosome. The parasites replicate within the cytoplasm of the host cell. There is considerable variation in the mechanism of replication between species, ranging from binary fission to 'schizogony'-like processes (sometimes called merogony). Some microsporidia replicate within the host cytoplasm, whereas others are found within parasitophorous vacuoles. Following replication the organisms differentiate into mature spores as exemplified by the appearance of the polar filament and thick spore wall. Mature spores are released from the host cell and are generally excreted with the feces or urine. Some microsporodia of insects are disseminated following the death of the host.
 |
 |
 |
 |
Reports of humans infected with microsporidia were extremely rare before the AIDS epidemic. Microsporidia are now recoginized as significant pathogens in the immuno-compromised host and clinical manifestations include intestinal, ocular, systemic and muscular diseases. Enterocytozoon bieneusi and three species of Encepalitozoon are the primary microsporidian species associated with humans (Table). Several other species have been sporadically reported.
| Microsprora Detected in Humans | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
*There are also several reports of Microporidium species which is a catch-all genus for Microspora which could not be classified. |
||||||||||||||||||
E. bieneusi is the most common microsporidia infecting humans and is a major cause of AIDS-related diarrhea. It primarily infects intestinal epithelial cells and causes a chronic diarrhea in AIDS patients. Dissemination to other sites is not common and primarily limited to the liver, gall bladder and lungs. In addition, E. bieneusi has been associated with self-limiting diarrhea in immunocompetent persons. E.bieneusi is found in other animals and pigs are a possible source of infection, but zoonotic transmission has not been documented. Human-to-human transmission via the fecal-oral route is probably the most important mode of transmission.
The three Encephalitozoon species are morphologically indistinguishable. They replicate by binary fission within a parasitophorous vacuole, in contrast to Entercytozoon, which forms multinucleated 'meronts' in direct contact with the host cytoplasm. The three species tend to produce different clinical manifestations, but all can infect macrophages and produce a disseminated disease. E. intestinalis, formerly called Septata intestinalis, is associated with a chronic diarrhea in AIDS patients and produces a disseminated disease less often. E. hellem is associated with a keratoconjunctivitis in both immuno-compromised and immunocompetent persons, but only produces disseminated disease in association with immune suppression. E. cuniculi infects a wide range of mammals and can infect several different organ systems.
|
Intestinal Microsporidiosis
Ocular Microsporidiosis
Systemic Microsporidiosis
Diagnosis
Treatment
|
The most common manifestation of microsporidiosis is diarrhea due to either E. bieneusi or E. intestinalis. AIDS patients with CD4+ counts <200/mm3 are especially prone to develop a chronic and debilitating diarrhea which leads to a wasting syndrome (cachexia). E. bieneusi usually remains localized and infects enterocytes of the small intestine (jejunum and duodenum). Pathology associated with infection includes: loss of enterocytes, villous atrophy (blunting), malabsorption, crypt hyperplasia and mononuclear cell infiltration. Encephalitozoon species initially infect enterocytes in the small intestine, causing intestinal cell damage and inflammation. These organisms can also infect macrophages in the lamina propria and disseminate throughout the body.
Ocular microsporidiosis is caused by several microsporidian species of which E. hellem is the most frequent. Immuno-competent persons usually exhibit keratitis or a corneal ulcer, which is possibly related to trauma; whereas a kerato-conjunctivitis is associated with AIDS. A systemic microsporidiosis is often manifested as a concomitant infection of the kerato-conjunctiva, urinary tract, and bronchia.
Diagnosis of microsporidiosis depends on the identification of spores in feces, urine, other bodily fluids, or within tissues. This can be difficult due to the small spore size and irregular spore excretion. A modified trichrome stain in which the chromotrope 2R component is increased is a common diagnostic method. Fluorochrome stains, such as calcofluor white, Uvitex 2B, or Fungifluor, bind chitin with a high affinity and can be used as a sensitive detection method. However, a fluorescent microscope is required. Biopsies should be carried out if spores are not detected in bodily secretions. There will be specificity problems in regards to identifying species at the light microscope level. Definintive diagnosis and speciation requires an electron microscope for the detection of the polar filament. However, electron microscopy is costly and time consuming, and thus not suitable for routine diagnosis.
Albendazole, a microtubule inhibitor, is currently the most effective drug for treating microsporidia infections. However, albendazole has a questionable effectiveness against E. bieneusi. A topical fumagillin is recommended for the treatment of kerato-conjunctivitis.
Reviews on microsporidia: