The apicomplexa are a monophyletic group composed almost entirely of parasitic (ie, no free-living) species. Apicomplexa, along with ciliates and dinoflagellates, form a higher order group known as Alveolata. A major defining characteristic of the this group are flattened vesicle-like structures--called cortical alveolae--which are found just underneath the plasma membrane. Formerly the apicomplexa were part of a group called sporozoa and this name is still sometimes used. There have been some suggestions to revert back to the name sporozoa (Cox, Tr. Parasitol. 18:108).
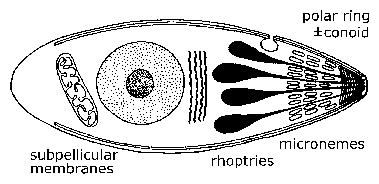
Electron microscopy revealed unique ultrastructural features among the various sporozoa which were subsequently used to redefine the groups. A defining characteristic of the apicomplexa is a group of organelles found at one end--called the apical end--of the organism. This 'apical complex' includes secretory organelles known as micronemes and rhoptries, polar rings composed of microtubules, and in some species a conoid which lies within the polar rings. At some point during their life cycle, members of the apicomplexa either invade or attach to host cells. It is during this invasive (and/or motile) stage that these apical organelles are expressed as well as the subpellicular membranes, which are actually cortical alveoli. The apical organelles play a role in interaction of the parasite with the host cell and the subsequent invasion of the host cell. (See detailed discussion on host cell invasion by malaria parasite.) Motile forms of apicomplexa crawl along the substratum in a non-ameboid fashion known as gliding motility. Many apicomplexan species have flagellated gametes.
 |
 |
| General Apicomplexan Structure and Life Cycle. Invasive and/or motile forms of apicomplexa exhibit distinctive ultrastructural features which can be seen with the electron microscope. At the very apical end is a ring of microtubules known as the polar ring. Sometimes an elaborate cytoskeletal structure known as the conoid is also seen. Small eliptical vesicles known as micronemes are also seen at this end as well as tear drop shaped organelles called rhoptries. | |
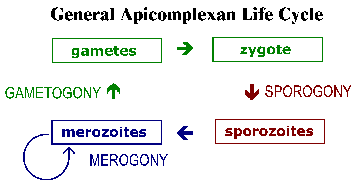
The apicomplexa have complex life cycles that are characterized by three distinct processes: sporogony, merogony and gametogony (Figure). Although most apicomplexa exhibit this overall general life cycle the details can vary between species. Furthermore, the terminology used to describe these various life cycle stages vary between the species. The life cycle consists of both asexually reproducing forms and sexual stages. In monoxenous species all three of these processes will be carried out in a single host and often in a single cell type or tissue. Whereas, in heteroxenous species the various processes will be carried out in different hosts and generally involve different tissues.
Sporogony occurs immediately after a sexual phase and consists of an asexual reproduction that culminates in the production of sporozoites. Sporozoites are an invasive form that will invade cells and develop into forms that undergo another asexual replication known as merogony. Merogony and the resulting merozoites are known by many different names depending of the species. In contrast to sporogony, in which there is generally only one round of replication, quite often there are multiple rounds of merogony. In other words, the merozoites, which are also invasive forms, can reinvade cells and initiate another round of merogony. Sometimes these multiple rounds of merogony will involve a switch in the host organism or a switch in the type of cell invaded by the parasite resulting in distinct stages of merogony. As an alternative to asexual replication merozoites can develop into gametes through a process variously called gametogony, gamogony or gametogenesis. As in other types of sexual reproduction, the gametes fuse to form a zygote which will undergo sporogony.
The apicomplexa are an extremely large and diverse group (>5000 named species). Seven species infect humans (Box). Plasmodium, as the causative agent of malaria, has the greatest impact on human health. Babesia is a relatively rare zoonotic infection. The other five species are all classified as coccidia. However, recent molecular data indicates that Cryptosporidium is more closely related to the gregarines than to the coccidia. The coccidia are generally considered opportunistic pathogens and are often associated with AIDS. Several apicomplexan parasites are also important in terms of veterinary medicine and agriculture. Most notable are Babesia and Theileria in cattle and Eimeria in poultry.
Historically the apicomplexa have been described as a group with only parasitic forms. This and their unique apical organelles bring up questions in regards to the origin of the group. Phylogenetic analysis indicates that members or the genus Copodella form a sister group with the apicomplexa (1). The colpodellids are predatory flagellates that feed on unicellular algae by a process called myzocytosis. Myzocytosis involves the predator (or parasite) attaching to the prey (or host) and literally sucking out the cytoplasm of the prey cell via specialized structures. This attachment and interaction with the prey cell is mediated by organelles similar to those that the apicomplexa utilize for attachment to or invasion of host cells. Thus the evolution of the apicomplexa likely evolved from this myzocytoic predation to myzocytoic parasitism, as exhibited by gregarines and Cryptosporidium, to intracellular parasitism. Other myzocytoic organisms with apicomplexa like apical organelles include Perkinsus, parasites of oysters and clams, and Parvilucifera, a predator of dinoflagellates. These perkinsids, however, form a sister group with the dinoflagellates and not the apicomplexa (Figure). This suggests that the progenitor of dinoflagellate and apicomplexan clades may have been a predatory flagellate and that the apical organelles were retained in the apicomplexan clade, but lost in most of the dinoflagellate clade. 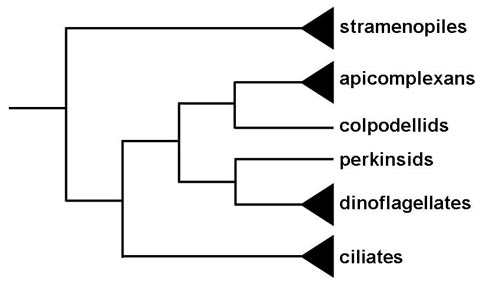
The other connection between algae and the apicomplexa is a chloroplast remnant, called the apicoplast, found in most apicomplexans (2). The apicoplast is likely the result of a secondary endosymbiosis of a red algae and is likely the same endosymbiotic event giving rise to the plastids of dinoflagellates. The apicoplast is nonphotosynthetic but exhibits activities associated with type II fatty acid biosynthesis, isoprenoid biosynthesis, and possibly heme synthesis. These pathways are essentially prokaryotic and represent excellent drug targets. A photosynthetic alveolate, Chromera velia, that appears to be the earliest branching apicomplexan has also been identified (3).
|
 |
 |
 |
 |
The coccidia are characterized by a thick walled oocyst stage that is typically excreted with the feces. Some coccidia (Cryptosporidium, Cyclospora, Isospora) carry out their entire life cycle within the intestinal epithelial cells of the host and are transmitted by the fecal-oral route. Other coccidia (Sarcocystis, Toxoplasma) have a more complicated life cycle involving tissue cysts and multiple hosts (ie, heteroxenous).
Since its initial identification in 1907 several Cryptosporidium species have been identified in a wide variety of animals ranging from fish to humans. The first human cases of cryptosporidiosis were reported in 1976 and were characterized as a diarrheal disease associated with immune suppression. Initially it was believed to be a rare and exotic disease. During the 1980’s Cryptosporidium was recognized as a major cause of diarrhea in AIDS patients and often resulted in death. However, it is now recognized that Cryptosporidium is a common cause of diarrhea in immunocompetent persons and has probably been a human pathogen since the beginning of humanity. Two species infecting humans have been identified: C. parvum and C. hominis.
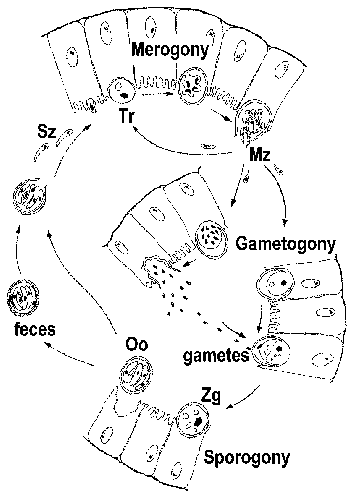
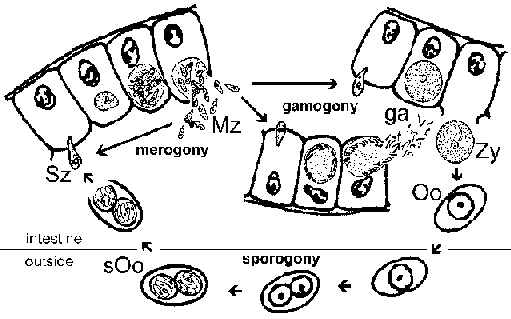
Cryptosporidium is often classified as a coccidian and exhibits a life cycle similar to other intestinal coccidia. However, Cryptosporidium is more closely related to the gregarines and this is reflected in some aspects of its life cycle. The infection is acquired through the ingestion of sporulated oocysts (Figure). [See larger figure of life cycle with detailed legend.] The pH changes associated with passage through the gut and bile and pancreatic fluids in the small intestine trigger excystation. Sporozoites (Sz) emerge from the oocyst and attach to intestinal epithelial cells. In contrast to other coccidia, Cryptosporidium sporozoites do not invade the enterocytes. Instead they induce the fusion and expansion of the microvilli resulting in the parasite becoming surrounded by a double membrane of host origin. A junction, called the 'feeder organelle' or the 'adhesion zone', forms between the parasite and the host enterocyte. The parasite, now called a trophozoite (Tr), likely derives nutrients from the host cell via this junction. (For a review on the 'invasion' process see Borowski et al, 2008.)
Trophozoites undergo an asexual replication (ie, merogony) and produce 4-8 merozoites (Mz) which are released into the intestinal lumen. The merozoites infect new intestinal epithelial cells and undergo additional rounds of merogony. The increased severity of the disease in immunocompromised patients is due in part to their inability to limit these additional rounds of merogony.
As an alternative to merogony, the merozoites can develop into either macro- or microgametocytes following the infection of an enterocyte. Microgametogenesis involves several rounds of replication followed by the release of numerous microgametes into the intestinal lumen. The microgametes fertilize macrogametes still attached to the intestinal epithelial cells. The resulting zygote (Zg) undergoes sporogony and the sporulated oocysts (Oo) are excreted with the feces. An autoinfection is also possible and this too may contribute to the increased disease severity in immunocompromised patients.
The risk factors of transmission for Cryptosporidium are similar to other fecal-oral diseases. However, waterborne cryptosporidiosis outbreaks have been especially notable. The most infamous is an outbreak in Milwaukee during the spring of 1993 in which an estimated 400,000 people developed symptomatic cryptosporidiosis (MacKenzie et al, New Eng. J. Med. 331:161, 1994). Factors that contribute to the increased risks of Cryptosporidium waterborne outbreaks are:
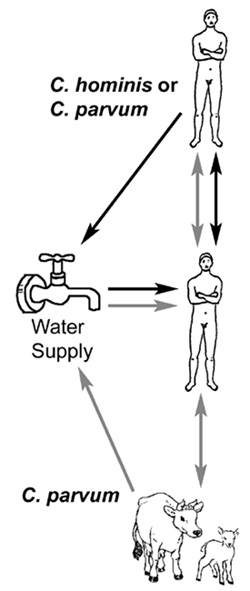
Despite the impressiveness of some waterborne outbreaks, human-to-human transmission appears to predominate. For example, asymptomatic infected children are common, secondary cases in households are high, and outbreaks tend to occur in hospitals, institutions and day care centers--situations typical for fecal-oral transmission. Molecular studies have revealed two primary genotypes isolated from humans. Genotype 1 has only been isolated from human sources and is non-infective for mice and calves. Genotype 2 has been isolated from both animal (bovine and ovine) and human sources and is infective for mice and calves. Based upon these and other biological differences it has been proposed to rename genotype 1 as Cryptosporidium hominis (Morgan-Ryan et al, J. Euk. Microbiol. 49:433, 2002). Other species and genotypes of Cryptosporidium (eg., C. felis, dog-like genotype, etc) have been isolated from AIDS patients and infrequently from immunocompetent humans (Morgan et al, J. Clin. Microbiol. 38:1180, 2000). A third species from the Indian subcontinent, C. viatorum, has also been proposed (Elwin et al, Int. J. Parasitol. 42:675, 2012).
Genetic data imply that there are two distinct transmission cycles in humans involving two different populations of Cryptosporidium: 1) an exclusively anthroponotic (ie, human-to-human) cycle caused by genotype 1 (or C. hominis) and 2) a zoonotic cycle caused by genotype 2 (or C. parvum). The zoonotic cycle would initially involve transmission from animals (eg, cattle or sheep) to humans and then subsequently human-to-human transmission and possibly a human-to-animal transmission. Both genotypes have been demonstrated to be the etiological agent in waterbourne outbreaks. Waterborne outbreaks linked to C. hominis are likely due to contamination of water with human sewerage, whereas waterborne outbreaks associated with C. parvum (genotype 2) are likely due to contamination of water with cow or sheep feces.
The most common clinical manifestation of cryptosporidiosis is a mild to profuse watery diarrhea. This diarrhea is generally self-limiting and persists from several days up to one month. Recrudescences are common. Abdominal cramps, anorexia, nausea, weight loss and vomiting are additional manifestations which may occur during the acute stage. The disease can be much more severe for persons with AIDS which manifests as a chronic diarrhea lasting for months or even years. Some AIDS patients exhibit a fulminant cholera-like illness which requires intravenous rehydration therapy. The fatality rate can be quite high in these fulminant cases.
Diarrhea can have osmotic, inflammatory, or secretory components (see Box). The watery nature of the diarrhea associated with Cryptosporidium infections has suggested the presence of an enterotoxin. However, there is no evidence for a toxin-mediated secretory diarrhea despite efforts to identify such a toxin. Experimental evidence does suggests that glucose-coupled Na+ absorption is decreased and Cl- secretion is increased. Therefore, the diarrhea associated with Cryptosporidium appears to be primarily osmotic in nature (see Figure). Associated with this disruption of enterocyte (i.e., intestinal epithelial cells) function is a blunting of the villi and crypt cell hyperplasia. A possible mechanism of pathogenesis is that the infection of intestinal epithelial cells with Cryptosporidium damages the enterocytes and eventually leads to their death. This triggers cell division in the crypt region (i.e., hyperplasia) to replace the damaged cells. The combination of destruction of absorbtive cells at the tips of the villi and the increase in the Cl--secreting crypt leads to an overall enhanced secretion.
|
|
In addition, an increased intercellular permeability and inflammation in the submucosal layer (aka, lamina propria) has been associated with Cryptosporidium infection. These phenomenon could also contribute to the secretory process via cytokines and neurohormones. For example, macrophages secreting tumor necrosis factor-alpha (TNF-α) or other cytokines may stimulate fibroblasts and other cells in the lamina propria to secrete prostoglandins (PGE) and other products (eg., reactive oxygen intermediates). These products may then promote secretion and impair absorption.
The parasite exhibits a trophism for the jejunum and ileum in immunocompetent persons, whereas the infection is more wide spread in AIDS patients and can include the stomach, duodenum, colon and biliary tract. This more extensive anatomical range in AIDS patients is presumably due to the inability of the immune system to control and limit the infection. Cell-mediated immunity appears to be the major component of the immune response in eliminating the infection as evidenced by the correlation between lower CD4+ T-cells and the risk and severity of cryptosporidiosis. Interferon-gamma, interleukin-12, and tumor necrosis factor-alpha are involved in protection against Cryptosporidium infection.
 |
 |
 |
 |
Isospora belli is believed to be a valid species which only infects humans. It has a worldwide distribution but is more common in tropical regions and areas with poor sanitation. Infections are often asymptomatic and those with symptoms tend to be self limiting with a duration of a few weeks. Infections are more common and the symptoms more severe in AIDS patients that in immunocompetent persons.
Life cycle. The infection is acquired through the ingestion of sporulated oocysts (sOo). Sporozoites (Sz) are release in the intestinal lumen and invade intestinal epithelial cells. Within the epithelial cells the parasite undergoes a round of merogony leading to the production of merozoites (Mz). The released merozoites reinvade intestinal epithelial cells and can undergo additional rounds of merogony or develop into either micro- or macrogamonts. Microgametes (ga) will fertilize the macrogametes (ga) to form a zygote (Zy) which develops into the oocyst (Oo). Immature oocysts are passed in the feces and maturation into infectious sporulated oocysts occurs in the environment. Recognizable stages during this maturation (ie, sporogony) include oocysts with a single sporoblast, oocysts with two sporoblasts, and the mature oocyst with two sporocysts, each of which contains four sporozoites. [See also detailed discussion of Cryptosporidium life cycle.]
Symptoms and Pathogenesis. Symptoms associated with I. belli infection include diarrhea, steatorrhea, headache, fever, abdominal pain, nausea, dehydration and weight loss. Blood is rarely present in the feces. In general, the symptoms are similar to those of cryptosporidiosis. The disease is often self-limiting. However, it can become chronic with oocysts being detected in the feces for months to years and recrudescences of the symptoms. The disease tends to be more severe in infants and young children than adults. Pathology associated with I. belli infections are primarily villous atrophy, or blunting, and crypt hyperplasia as commonly seen in other intestinal infections.
The diarrhea in AIDS patients is often very watery and can lead to dehydration requiring hospitalization. Fever and weight loss are also a common finding. Another common finding among AIDS patients is a chronic intermittent diarrhea lasting for months to years. The resulting excessive weight loss and electrolyte imbalance can lead to wasting and even death. There have also been a few reports of disseminated extraintestinal isosporiasis in AIDS patients.
Lindsay, DS, Dubey, JP, Blagburn, BL (1997) Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin. Microbiol. Rev. 10: 19-34.
The first human cases of Cyclospora cayetanensis were reported in 1979. It was originally referred to as cyanobacteria-like bodies or coccidian-like bodies (CLB). The organism was confirmed to be a coccidian parasite with an oocyst structure similar to the genus Cyclospora and then named in 1994 after the Universidad Peruana Cayetano Heredia in Peru where most of the early studies had been carried out. Molecular studies indicate a close relationship to Eimeria, an important veterinary parasite of poultry and other livestock. C. cayetanenis has a worldwide distribution, but appears to be especially prevalent in Latin America, the Indian subcontinent, and southeast Asia. In developed countries the infections are usually associated with either foodborne outbreaks or traveller’s diarrhea.
Life cycle and transmission. The life cycle of Cylcospora is similar to Isospora (see above). The infection is acquired through the ingestion of oocysts. Sprorozoites are released and infect epithelial cells of the upper small intestine. The parasite undergoes merogony and the merozoites reinfect enterocytes and several more rounds of merogony can occur. Some of the merozoites undergo a sexual development resulting in the production of micro- and macrogametes. Fertilization of the macrogamete by the microgamete initiates sporogony and the formation of the oocyst. Like Isospora, sporulation is completed in the environment and immature non-infectious oocysts are excreted in the feces. The maturation of the oocysts to sporulated infectious oocysts probably takes days to weeks. In addition, the structure of the Cyclospora oocyst is different from that of Isospora. The oocyst contains two sporocysts which each contain two sporozoites.
Several outbreaks in the United States and Canada have been associated with fresh produce imported from South and Central America (Table). In particular, berries and leafy vegetables have been identified as the probable contaminated item. These are food items that are typically eaten raw and are only rinsed. No outbreaks have been associated with frozen, processed, or peeled fruits or vegetables. A seasonality in the outbreaks has also been observed with most of the cases occurring in the spring and early summer. A similar seasonality has been observed in endemic countries also. In contrast to the United States and Canada, where foodborne transmission dominates, the majority of the cases in Europe and Australia have been associated with travel to endemic countries.
Selected Cyclosporiasis Outbreaks in United States and Canada |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As a result of the high number of outbreaks associated with raspberries from Guatemala the United States restricted the import of raspberries and required inspection of farms. This resulted in a subsequent drop in the number of outbreaks in the United States. Canada, which did not restrict importations, did not experience a drop in the number of outbreaks during this period. Subsequent case controlled studies carried out in Guatemala revealed that infections were most common in children with the prevalence peaking in June. The major risk factor associated with the infection was drinking untreated water. In Peru contact with soil was identified as another risk factor, especially among children less than two years old. Inadequate water treatment in these endemic countries may lead to contamination of the ground water and thus maintain the transmission cycle. Presumably foodborne transmission is due to irrigation or applying fertilizer with contaminated water or washing and processing foods with poorly treated water.
Symptoms. Cyclospora primarily infects epithelial cells in the upper portion of the small intestine. The incubation period is generally one-two weeks. Symptoms are similar to the gastroenteritis caused by Isospora and Cryptosporidium which typically includes cycles of watery diarrhea and periods of apparent remission. The diarrhea is characterized by frequent stools and can persist for up to six weeks, but is generally self-limiting in immunocompent persons. Anorexia, malaise, nausea and cramping are other frequent symptoms associated with cyclosporiasis. In some cases patients may experience vomiting, muscle aches, substantial weight loss, and explosive diarrhea. Previous exposure to Cyclospora appears to confer some resistance to infection with a lessening of the symptoms. Over time adults appear to develop immunity and asymptomatic carriers can be found in endemic areas.
As is also
the case for Cryptosporidium and Isospora, the diarrhea caused
by Cyclospora in AIDS patients is much more severe than in immunocompetent
persons. The diarrhea can last for months and produce a syndrome that is debilitating
and life threatening.
Cyclospora reviews:
Sterling and Ortega (1999) Clycospora: an enigma worth unraveling. Emerg. Inf. Dis. 5:48.
BL Herwaldt (2000) Cyclospora cayetanensis: A review focusing on the outbreaks of clyclosporiasis in the 1990s. Clin. Inf. Dis. 31:1040.
JM Shields and BH Olson (2003) Cyclospora cayetanensis: a review of an emerging parasitic coccidian. International Journal for Parasitology 33, 371-391.
LS Mansfield and AA Gajadhar (2004) Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Veterinary Parasitology 126: 73-90.
YR Ortega and R Sanchez (2010) Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 23:218-234.
 |
 |
 |
 |
Coccidiosis is diagnosed by demonstrating oocysts in the feces. Acid-fast staining is the preferred method for coccidia which stain bright red. Cryptosporidium, Cyclospora, and Isospora are distinguished by size and oocyst structure (Table). Cyclospora and Isospora do not uniformly take up the stain resulting in a mixture of unstained, partially stained and completely stained oocysts. Cyclospora and Isospora can also be detected via an autofluorescence associated with the cyst wall. Because of its relatively large size, Isospora is readily detected in unstained specimens. Sarcocystis is a rare human infection (see below) with oocysts similar to Isospora except that the sporocysts are generally released from the oocysts while still in the intestinal lumen.
| Coccidian Parasites Found in Human Feces | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The recommended treatment for Cyclospora and Isospora is the combination of trimethoprim-sulfamethoxazole (Bactrim). There is no completely satisfactory treatment for Cryptosporidium. It is hypothesized that the 'extracytoplasmic' location of Cryptosporidium shelters it from drugs. Paromomycin has been used for the treatment of cryptosporidiosis, however, its efficacy is debated. Controlled studies indicate that paromomycin modestly suppresses parasitemia in immunocompromised individuals. Treatment of severe cryptosporidiosis should include supportive care (rehydration and nutritional support) and anti-motility agents. Preventive measures will be similar to other diseases transmitted by the fecal-oral route (see risk factors or Giardia control).
 |
 |
 |
 |
Some coccidian species exhibit a heteroxenous life cycle in which merogony takes place in tissues of the intermediate host (prey) and gametogony takes place in the intestinal epithelium of the definitive host (predator). [By convention, sexual reproduction occurs in definitive hosts.] In regards to human infections, Toxoplasma is a common tissue cyst forming coccidian parasite, whereas infections with Sarcocystis species are quite rare.
The life cycle (see Figure) of Sarcocystis within the predator (ie, carnivore) is similar to the life cycles of intestinal coccidia, such as Isospora, involving a sexual cycle (gametogony) within the intestinal epithelial cells. One difference in the Sarcocystis life cycle is the lack of merogony in the intestinal epithelial cells. In other words, the merozoites acquired by ingesting an infected prey will only produce gametes following the invasion of intestinal epithelial cells. Fusion of the gametes leads to the production of oocysts. In addition, the sporocysts are usually released from the oocysts within the intestine of the host and therefore infectious sporocysts are found in the feces.
The intermediate hosts (herbivore) acquire the infection by ingesting the sporulated sporocysts. Sporozoites are released, invade intestinal epithelial cells, and undergo merogony as is typical of intestinal coccidia. In contrast to intestinal coccidia, the merozoites will invade endothelial cells and produce a systemic infection. Quite often there is a tropism for particular tissues such as brain or muscle. The meronts (or schizonts) in these tissues are often often encapsulated and referred to as 'tissue cysts'. These tissue cysts, or sarcocysts in the case of muscle, often exhibit a lower level of replication during merogony and are somewhat dormant. Ingestion of the infected animal by a carnivore will release the merozoites which will invade intestinal epitheial cells and thus completing the life cycle.

This predator-prey life cycle was not worked out until the 1970's. Previously, the intestinal infections in the predator were usually designated as Isospora species and the tissue infections in the prey were usually designated as Sarcocystis species. In many cases the definitive hosts have not been positively identified and the taxonomy of many Sarcocystis species is uncertain.
Sarcocystis infections in humans have been documented, but are rare. Humans are the definitive host for S. hominis (aka, S. bovihominis) and S. suihominis as determined by the source of the infection being either beef or pork, respectively. Ingestion of undercooked beef or pork from infected animals will produce an enteric infection which can produce acute intestinal symptoms (abdominal discomfort, nausea, diarrhea). However, most infections are believed to be asymptomatic. Infected individuals can shed sporocysts in the feces for weeks to months following the infection. Sporocysts from human feces are infective to cows, pigs and deer.
Humans can also serve as the intermediate host to at least some of the Sarcocystis species found in nature. Ingestion of the sporocysts by humans can result in the tissue stage of the infection and the formation of sarcocysts. These sarcocysts are generaly several 100 µm in size and cause little tissue damage. Clinical symptoms can include muscle tenderness or episodic painful inflammatory swellings. These muscle cysts in humans have only been sporadically reported (<100 reported cases) and probably represent accidental infections. One study noted that the sarcocysts in humans tended to resemble Sarcocystis species commonly found in local monkeys (Beaver et al, Am. J. Trop. Med. Hyg. 28:819, 1979). Most of the cases have been reported from tropical and subtropical Asia, including an outbreak of muscular sarcocystosis among travelers returning from Malaysia (MMWR 61:37).
Fayer, R (2004)
Sarcocystis spp. in Human Infections. Clin.
Microbiol. Rev. 17: 894-902.
 |
 |
 |
 |
Toxoplasma gondii is a coccidian parasite which infects humans as well as a wide variety of mammals and birds. It exhibits a predator-prey type life cycle (as discussed above for Sarcocystis) and felines are the only definitive host. Toxoplasmosis is found throughout the world (except extremely cold or dry climates) and tends to be more prevalent in tropical climates. Serologic studies have shown prevalence rates up to 70% by the age of 25 in some central American populations. In the United States an estimated 0.5-1% of the population becomes infected each year and prevalence ranges from 10-25% by the age of 25. Toxoplasmosis is most often a benign disease. Noted exceptions are in the cases of congenital infection or immunocompromised individuals.
Toxoplasma has a complex life cycle consisting of intestinal and tissue phases. Although the organism was first discovered in 1908 as a tissue parasite of the gondi (an African rodent), its complete life cycle was not determined until 1970. The intestinal phase of the infection occurs only in felines and exhibits a typical intestinal coccidian life cycle consisting of merogony and gamogony (see Isospora life cycle). Cats acquire the infection by eating animals infected with the tissue stage of the parasite. The parasites invade intestinal epithelial cells and undergo merogony. The resulting merozoites can then either undergo additional rounds of merogony or undergo gametogony. As is similar to other apicomplexa (see general apicomplexan life cycle) macro- and microgametes are produced. Thus, the cat is considered the definitive host since this is the host in which the sexual cycle occurs.
The bi-flagellated microgametes are released into the lumen of the intestine and fertilize the macrogametes within the host epithelial cells. Secretion of the oocyst wall begins shortly after fertilization. This sexual cycle culminates in the production of oocysts which are excreted in the feces. These immature oocysts undergo sporogony at ambient temperature resulting in mature oocysts containing two sporocysts, each with four sporozoites. Sporulation generally takes 1-4 days and the oocysts remain infective for months in shaded moist soil. It has been hypothesized that unfertilized macrogametes may also be capable of forming mature oocysts (Ferguson, Tr. Parasitol. 18:351, 2002).
 Intermediate
hosts, such as rodents and birds, become infected through the ingestion of sporulated
oocysts. Sporozoites are released, penetrate the intestinal epithelium, and
invade macrophages and other types of cells. The invasion process is typical
of apicomplexa and the parasite lies within a parasitophorous vacuole. Within
the vacuole the parasite undergoes binary fission (i.e., merogony) by a unique
process called endodyogeny. Endodyogeny is a specialized type of division in
which the two daughter cells form within the mother cell. These trophic forms
are called tachyzoites (tachy means rapid) in reference to their high
level of replication. The host cell will rupture and release the tachyzoites
which will invade new host cells and repeat the replicative cycle. Infected
macrophages will disseminate the tachyzoites throughout the host during this
acute infection.
Intermediate
hosts, such as rodents and birds, become infected through the ingestion of sporulated
oocysts. Sporozoites are released, penetrate the intestinal epithelium, and
invade macrophages and other types of cells. The invasion process is typical
of apicomplexa and the parasite lies within a parasitophorous vacuole. Within
the vacuole the parasite undergoes binary fission (i.e., merogony) by a unique
process called endodyogeny. Endodyogeny is a specialized type of division in
which the two daughter cells form within the mother cell. These trophic forms
are called tachyzoites (tachy means rapid) in reference to their high
level of replication. The host cell will rupture and release the tachyzoites
which will invade new host cells and repeat the replicative cycle. Infected
macrophages will disseminate the tachyzoites throughout the host during this
acute infection.
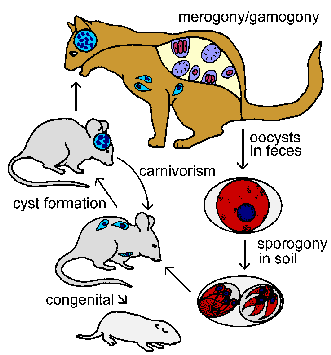
As the host develops immunity the replication rate will slow and the infected host cells will become encapsulated (ie, tissue cysts). These slowly replicating forms are called bradyzoites (brady means slow) and represent a dormant or resting stage. Bradyzoites are considered to be metabolically quiescent, but remain viable (Dubey et al, Clin. Microbiol. Rev. 11:267, 1998). Other changes that occur when tachyzoites convert to bradyzoites include secretion of chitin and other components to form a cyst wall and the accumulation of amylopectin granules (reflecting glucose storage). The tissue cysts of Toxoplasma exhibit a variety of sizes, but often obtain a size of 50-70 µm in diameter containing 1000-2000 bradyzoites. The bradyzoites are primarily found in brain and muscle tissue, whereas the tachyzoites tend to be in reticuloendothelial cells.
The bradyzoite stage represents a chronic infection and probably persists for the life of the host. The mechanism for this persistence is unknown. Some investigators believe the tissue cysts periodically break down and release the bradyzoites which will invade new host cells and lead to the formation of more tissue cysts.The tissue phase of the infection can also be transmitted congenitally to offspring and to other intermediate hosts through carnivorism. Ingestion of an infected animal will release the bradyzoites from the tissue cysts which then infect cells in the new host. Possibly all mammals, including humans, can become infected with Toxoplasma. As in the case of acquiring the infection through the ingestion of oocysts, the parasites will go through an acute phase characterized by rapid replication followed by a chronic phase characterized by dormant tissue cysts. Ingestion of an infected intermediate host by the cat will initiate the intestinal stage of the life cycle involving merogony and gamogony in the intestinal epithial cells. Cats can also support the tissue stage of the infection.
| Human Transmission |
|---|
|
Obviously humans are not a natural part of the predator-prey life cycle and represent an accidental host that does not participate in the continuation of the transmission cycle. One source of infection is the ingestion of material contaminated with sporulated oocysts excreted by cats. This implies some association with cats. However, since the oocysts need to mature in the environment before becoming infectious, transmission will include features of soil transmission similar to Isospora and Cyclospora. For example, children of crawling and dirt-eating ages are believed to be at higher risk for infection. Oocysts can also be acquired through gardening activities or unwashed fruits or vegetables. In addition a few water-borne outbreaks have been documented. The high prevalence of toxoplasmosis in South and Central America is believed to be due to high levels of contamination of the environment with oocysts. Ironically, contact with dogs is more of a risk factor for becoming infected with Toxoplasma than contact with cats. This is likely due to dogs seeking out cat feces and becoming contaminated with the feces and then transferring the sporulated oocysts to the clothes and hands of their owners. Interestingly, a waterborne outbreak associated with kittens living on top of a municipal water reservoir in Brazil was reported (de Moura et al, 2006, Emerg. Inf. Dis. 12:326).
Toxoplasmosis can also be acquired through the ingestion of undercooked meat containing tissue cysts or tachyzoites. Presumably livestock acquire the infection through grazing in areas contaminated with cat feces. The ability of the parasite to transfer between intermediate hosts may be a relatively recent evolutionary adaptation of the parasite that coincides with the domestication of the cat and the expansion of agriculture (see Box). In fact, most infections in the United States and Europe among adults are probably acquired from undercooked meat. The especially high seropositive rate in France (up to 90%) is likely due to a cultural predilection for lightly cooked or raw meat. Mutton and pork are more common sources than beef. There have also been a few isolated reports of Toxoplasma being transmitted via tachyzoites in unpasteurised goat's milk.
Toxoplasma can also be transmitted from mother to fetus, often with dire consequences (see below). Congenital transmission can only occur during an acute infection (ie, tachyzoites) acquired during pregancy. Mothers with a chronic infection acquired before the pregnancy are not at a risk for transmitting Toxoplasma. Tranmission of Toxoplasma as a result of organ transplants is also possible. Tissue cysts from a chronically infected organ donor may reactivate when transplanted into a previously uninfected organ recipient. In addition, the immunosuppressive therapy could also reactivate a latent infection in the recipient. Acquisition of tachyzoites from an acutely infected person via blood transfusion is also possible. Transmission by transplantation or transfusion are now rare, though.
| Molecular
analysis of Toxoplasma isolates (primarily from North America and
Europe) reveal a limited genetic diversity. The majority (>94%) of the
isolates cluster into three distinct clonal lineages designated as Type
I, Type II and Type III. These three clonal lineages are closely related
and are comprised of various mixtures of just two alleles at the loci tested.
The three types may have arisen from genetic recombination occurring within
the last 10,000 years (1). This would be concurrent with the expansion of
human agriculture and the adaptation of the domestic cat. Thus, changes
in human behavior may have led to a selection and rapid propagation of Toxoplasma.
Furthermore, these clonal types all exhibit the ability to be transmitted
via a direct oral route between intermediate hosts, which may have not been
a biological feature of the ancestral Toxoplasma or other closely related
species like Neospora (see box below).
This acquisition of direct oral infectivity combined with domestication
of animals could promote a rapid, and primarily asexual, expansion of Toxoplasma.
The three Toxoplasma genotypes also exhibit differences in virulence (2). For example, type I parasites are highly virulent in mice. Similarly, type I is disproportionately associated with severe atypical ocular toxoplasmosis in immunocompetent individuals and severe congenital toxoplasmosis. Fortunately though, type II infections tend to dominate, especially in the U.S. However, there is some evidence suggesting that Toxoplasma exhibits more diversity in South America. Improvements in our knowledge about Toxoplasma population biology may help resolve these issues and lead to better control and treatment.
|
 |
 |
 |
 |
Toxoplasmosis in adults and children past the neonatal stage is usually benign and asymptomatic. Acquisition of the infection via either oocysts or tissue cysts results in an acute infection in which tachyzoites are disseminated throughout the body via the lymphatics and hematogenously. This acute stage will persist for several weeks as immunity develops. Antibody production requires 1-2 weeks and cellular immunity occurs 2-4 weeks post-infection. Both humoral and cellular immunity are important, but the cellular response appears critical for the conversion from acute (ie, tachyzoites) to chronic (ie, bradyzoites) infection. (See life cycle for explanation of tachy- and bradyzoites.) In particular, a strong Th1 response characterized by the production of proinflammatory cytokines including interleukin-12, interferon-gamma, and tumor necrosis factor-alpha are associated with Toxoplasma infection.
When symptoms do occur they are generally mild and typically described as mononucleosis-like with chills, fever, headache, myalgia, fatigue and swollen lymph nodes. These symptoms are self-limiting and resolve within weeks to months. A chronic lympadenopathy without fever persisting or recurring for up to a year has also been noted as a symptom of toxoplasmosis. Rarely do immunocompetent individuals exhibit severe symptoms and the acute infection almost always progresses to the chronic stage. This latent infection probably persists for the life of the patient without producing any progressive pathology.
| Toxoplasmic Encecphalitis |
|---|
|
Toxoplasmosis has been long noted as an opportunistic infection in regards to reactivation of latent infections due to immunosuppression associated with organ transplants and certain cancer treatments. During the 1980's toxoplasmic encephalitis emerged as a common complication associated with AIDS. An estimated 25-50% of AIDS patients with chronic toxoplasmosis will develop encephalitis. Reactivation of the infection typically occurs when CD4 cells drop below 100 cells per microliter. Early symptoms of toxoplasmic encephalitis can include headache, fever, lethargy, and altered mental status with progression to focal neurological deficits and convulsions. The disease is almost always due to a reactivation of a latent infection (see Box) and tends to remain confined to the CNS. In other words, the tissue cysts are rupturing and the released bradyzoites are transforming into tachyzoites. (See life cycle for explanation of tachy- and bradyzoites.) The focal lesions are caused by the destruction of host cells in the immediate vicinity. Other forms of the reactivated disease, especially retinochoroiditis, pneumonitis, myocarditis and myositis, may occasionally occur in conjunction with immunosuppression.
Carlos S. Subauste, Toxoplasmosis and AIDS.
| Congenital Infection Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Toxoplasma can also be transmitted congenitally (i.e., transplacentally) if the mother acquires the infection during pregnancy. Congenital (ie, transplacental) infections are more likely to be symptomatic than postnatal infections and can be particularly severe (see Outcomes Box). Some of the salient features are:
A retinochoroiditis, inflammation of the retina and choroid (thick vascular area at back of eye), is another clinical manifestation of Toxoplasma infection. Retinochoroiditiis can result from congenital infections or from acute or reactivated infections acquired postnatally. Originally the ocular manifestations were more often associated with congenital infections or a late manifestation due to the reactivation of a congenital infection. However, ocular toxoplasmosis is being reported with increasing frequency in association with acute infections. It has been suggested that different genotypes exhibit different levels of virulence especially in regards to the expression of ocular disease. In the case of congenital infection, the retinochoroiditis can develop weeks to years after birth. Approximately twenty percent of persons with congenital infections will exhibit retinochoroiditis at birth and by adolescence 82% will exhibit symptoms.
The lesions are focal in nature and generally self-limiting. They are believed to be the result of cyst rupture in the retina in reactivated cases or tachyzoites in acute cases. The cells of the retina closely resemble those of the central nervous system. Granulomatous lesions may also be present in the choroid. The lesions are usually bilateral in congenital infections and unilateral if acquired postnatally. Animal studies provide evidence that the retinal necrosis associated with the lesion is attributable to proliferation of parasites, while hypersensitivity responses to toxoplasmic antigens are responsible for the accompanying inflammation. Symptoms can include blurred vision or other visual defects. Vision may improve with the resolution of the inflammation. Recurrences of the disease have been noted, but the frequency and factors influencing the recurrence are not clear. The disease is rarely progressive in immunocompetent individuals, but can scar the retina. However, the disease can be quite severe in AIDS patients and continue to progress.
 |
 |
 |
 |
In contrast to most other protozoan infections, diagnosis is rarely made through the detection or recovery of organisms, but relies heavily on serologic procedures. Parasites can be detected in biopsied specimens, buffy coat cells, or cerebral spinal fluid. However, detecting tachyzoites from these materials may be difficult. These specimens can also be used to inoculated mice or tissue culture cells or analyzed by PCR. The results can be misleading though, since many individuals have been exposed to Toxoplasma and harbor tissue cysts (bradyzoites). Therefore, serologic tests are a recommended component of diagnosis.
The serologic diagnosis of Toxoplasma is also complex because of the prevalence of sero-positive individuals. High antibody titers by themselves are not definitive evidence of an acute infection. Congenital infections are similarly difficult to diagnose serologically because maternal IgG crosses the placenta and persists for several months. Evidence for an acute infection are high IgM titers and/or significant increases in total antibody titers in conjunction with symptoms. Imaging techniques (CT, MRI) may also be useful in the diagnosis of toxoplasmic encephalitis.
Treatment indications and duration:
The recommended treatment is the synergistic combination of pyrimethamine plus sulfadiazine supplement with folinic acid (Leucovorin®).
| Prevention |
|---|
raw meat
|
The prognosis for acute toxoplasmosis in immunocompetent adults is excellent. Acute infections in the fetus or young children may be followed by repeated attacks of retinochoroiditis. Treatment does appear to reduce the frequency of these attacks. If begun early enough, treatment of immunosuppressed patients usually results in improvements, but recrudescences are common.
Control measures for toxoplasmosis focus on avoiding the two major sources of infection: raw meat and contaminated cat feces. Preventive activities (Box) include: avoiding the ingestion of sporulated oocysts or tissue cysts, destruction of the infective forms (eg., heating), and preventing the infection of pets. Prevention is especially important during pregnancy when the consequences of infection are most severe.
Reviews on Toxoplasma:
| Neospora caninum is closely related to Toxoplasma gondii and exhibits a nearly identical morphology. As with Toxoplasma, Neospora infects many domestic animals and is a major cause of abortions and stillbirth in cattle worldwide. The definitive hosts are dogs which exhibit neuromuscular disease. Humans are not a host. Dogs become infected after ingestion of infected tissues from intermediate hosts. Unsporulated oocyts are shed in the feces and sporulate in the environment. Intermediate hosts acquire the infection by ingestion of sporulated oocysts. However, unlike Toxoplasma, intermediate hosts cannot acquire the infection by ingestion of tissue forms from other intermediate hosts. The infection can be transmitted congenitally and the parasite is readily maintained in cattle and dogs by vertical transmission. A sylvatic cycle involving white-tailed deer and coyotes has also been identified (Rosypal and Lindsay, Tr. Parasitol. 21, 349. 2005). |
 |
 |
 |
 |
| Historical Note |
|---|
| In 1893, Smith and Kilborne reported that ticks tranmit B. bigemina, the cause of Texas cattle fever. This was the first demonstration of an arthropod transmitted disease and likely inspired the subsequent discovery of other vector-transmitted diseases such as yellow fever and malaria. |
Babesiosis is a rare zoonotic infection transmitted by ticks. The etiological agents, Babesia species, are blood parasites which infect a wide variety of wild and domestic animals throughout the world. Babesia and Theileria form a group called the piroplasms, in reference to intraerythrocytic forms that are pear-shaped in some species. Piroplasms cause tremendous losses of livestock in endemic areas. Is has been speculated that the plague of the Egyptians' cattle described in the biblical book of Exodus may have been red water fever caused by B. bovis.
The two piroplasm genera are usually distinguished by
the lack of a pre-erythrocytic cycle in Babesia
and the lack of transovarial transmission in Theileria
(see life cycle below).
Molecular data indicate that Babesia
and Theileria species
do not form respective monophylogenic groups. In particular, many Babesia
species, which had been informally grouped as 'small' Babesia, are more
closely related to Theileria. Consistent with this molecular data, none
of the small Babesia--in contrast to the 'large' Babesia--appears
to be transmitted transovarially in ticks, suggesting a need for some re-evaluation
of piroplasm classification. (See G Uilenberg, 2006, Babesia--A historical overview,
Veterinary
Parasitology 138, 3-10.)
*Peromyscus leucopus (white footed mouse) and Microtus pennsylvannicus, respectively. |
Many species of Babesia have been reported to infect humans. The three most predominant species infecting humans are B. microti, B. duncani, and B. divergens (Table). Infections with other species have either been poorly documented or limited to a few isolated cases. The initial cases were associated with splenectomy or other immuno-compromising conditions. However, immuno-competent persons infected with Babesia and not exhibiting clinical symptoms have been described. Furthermore serological surveys suggest that the infection may be under diagnosed. The largest focus of human infections in the U.S. has been along the northeastern costal region, giving rise to the name Nantucket fever, and the upper midwest. Infection in Europe is apparently rarer then in the U.S., but more fatal. Most of these infections have been associated with individuals who have frequent contact with cattle.
 |
 |
 |
 |
Babesia exhibits a typical apicomplexan life cycle characterized by merogony, gametogony, and sporogony (Figure). The infection is acquired by the vertebrate host when sporozoites (Sp) are transferred during tick feeding. The sporozoites invade erythrocytes utilizing a mechanism of invasion that is similar to other Apicomplexa. (See detailed discussion on host cell invasion by malaria parasite.) In contrast to Plasmodium, the parasitophorous vacuolar membrane (PVM) disintegrates after invasion and the parasite is in direct contact with the host erythrocyte cytoplasm. The trophozoites (Tr) divide by binary fission and produce merozoites (Mz), which infected additional erythrocytes and reinitiate the replicative cycle. In some species, a tetrad, referred to as a Maltese cross, is occasionally observed.
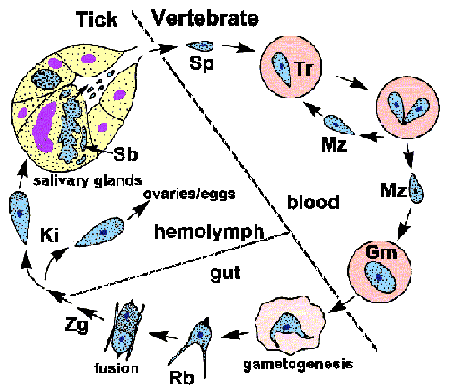
Some of the trophozoites will develop into gametocytes (Gm), or gamonts, which are responsible for initiating the infection in the tick vector. The gametocytes undergo morphological changes within the tick's gut and develop into ray bodies (Rb; aka Strahlenkörper). Two ray bodies (i.e., gametes) will fuse to form a zygote (Zg) which then develops into a kinete (Ki). The kinete penetrates the peritrophic membrane and intestinal epithelium to gain access to the hemolymph. Large Babesia, such a B. divergens and B. canis, are capable of invading various organs and undergoing further replication. Most notable is the invasion of the ovaries and eggs leading to a transovarial transmission to the tick's offspring.
Sporogony is initiated when kinetes invade the salivary glands. The parasite expands and fills a hypertrophied host cell and develops into a multinucleated sporoblast (Sb; aka sporont). Mature sporozoites, possessing apical organelles, will bud from this undifferentiated sporoblast when the tick feeds again on a new host. Five-ten thousand sporozoites can be produced by a single sporoblast. The sporozoites will then be injected into the host with the saliva, thus completing the life cycle.
| Theileria species infect and cause disease in livestock, and especially cattle, in many parts of the world. The most serious is East Coast fever of cattle, caused by T. parva. It has 90–100 percent mortality in Africa. T. annulata causes a milder disease of cattle along the Mediterranean and in the Middle East known as tropical theileriosis. Theileria are closely related to Babesia and exhibit a very similar life cycle (Figure 16.1). The major difference is a pre-erythrocytic stage exhibited by Theileria species. Although such a pre-erythrocytic stage is suspected in B. microti. Sporozoites invade lymphocytes and induce proliferation of the host lymphocytes by an unknown mechanism. The parasite develops into a multinucleated schizont (i.e., meront) which undergoes division coincident with the replication of the proliferating lymphocyte, and thus a schizont is transferred to each of the daughter lymphocytes. The resulting merozoites invade erythrocytes and ultimately develop into gamonts which are infectious for the tick. It is the lymphoproliferative process that leads to the severe disease manifestations associated with theilerioses. This lymphocyte transformation is reversible in that treatment leads to parasite clearance and subsequent lymphocyte proliferation is inhibited. |
 |
 |
 |
 |
In persons with intact spleens the infection is generally self-limited and characterized by a gradual onset of malaise, fever, headache, chills, sweating, myalgia, fatigue and weakness. A mild to moderate hemolytic anemia can also accompany these symptoms. Many of the infections will resolve on their own without treatment, but the parasites can persist for months. The disease tends to be more fulminant and severe in splenectomized or immunosuppressed individuals and can be life threatening. Parasitemias of >25% and severe anemia can occur.
Diagnosis is confirmed by detecting the parasite in Giemsa-stained blood smears.
There is a possibility of Babesia being confused with the malaria parasite
due to some morphological similarities to Plasmodium ring-stage parasites
(see Figure of B. microti). Serology, lack of response to anti-malarials,
and no travel history are other factors to consider in making the diagnosis.
There are no clearly effective drugs against babesiosis. The recommended treatment is clindamycin + quinine. Pentamidine has also been shown to suppress, but not eliminate, parasitemia. Chloroquine, although it does not appear to affect parasitemia, does provide some symptomatic relief, which may be due to its anti-inflammatory properties. Atovaquone + azithromycin has been shown to be as effective as clindamycin + quinine, but with fewer adverse affects (Krause et al, 2000, N. Engl. J. Med 343:1454). Exchange transfusion has been used as a life-saving effort in severely ill patients.
Review on babesiosis:
These pages are developed and maintained by Mark F. Wiser, Tulane University (©2000). Last update on March 19, 2024.