



| Historical Highlights | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Malaria has been and still is the cause of much human morbidity and mortality. Although the disease has been eradicated in most temperate zones, it continues to be endemic throughout much of the tropics and subtropics. Forty percent of the world's population lives in endemic areas. Epidemics have devastated large populations and malaria poses a serious barrier to economic progress in many developing countries. There are an estimated 300-500 million cases of clinical disease per year with 1.5-2.7 million deaths. Some of the earliest known medical writings from China, Assyria, and India accurately describe the malaria-like intermittent fevers. Hippocrates, the 'father of medicine', is generally credited with the first description of the clinical symptoms in 500 BC, more than 2000 years before the parasite was described (Table). [See also Wikipedia article on History of Malaria.]
Malaria is caused by members of the genus Plasmodium. Plasmodium species are apicomplexa (see general description of apicomplexa) and exhibit a heteroxenous life cycle involving a vertebrate host and an arthropod vector. Vertebrate hosts include: reptiles, birds, rodents, monkeys and humans. Plasmodium species are generally host specific and vector specific in that each species will only infect a limited range of hosts and vectors. Four distinct species infected humans: P. falciparum, P. vivax, P. ovale and P. malariae. (See page on species differences.) The species differ in regards to their morphology, details of their life cycles, and their clinical manifestations.
 |
 |
 |
 |
Human and other mammalian Plasmodium species are transmitted by anopheline mosquitoes. The parasite is injected with the saliva during mosquito feeding and first undergoes a round of merogony in the liver followed by multiple rounds of merogony in the erythrocytes. Gametogony begins within the erythrocytes of the vertebrate host and is completed within the mosquito where sporogony takes place. This life cycle exhibits the general features of other apicomplexan parasites characterized by asexual replication and the formation of invasive stages with typical apical organelles.
Liver Stage. Human infection is initiated when sporozoites are injected with the saliva during mosquito feeding. The sporozoites enter the circulatory system and within 30-60 minutes will invade a liver cell. Host cell entry, as in all apicomplexa, is facilitated by the apical organelles. After invading the hepatocyte, the parasite undergoes an asexual replication. This replicative stage is often called exoerythrocytic (or pre-erythrocytic) schizogony. Schizogony refers to a replicative process in which the parasite undergoes multiple rounds of nuclear division without cytoplasmic division followed by a budding, or segmentation, to form progeny. The progeny, called merozoites, are released into the circulatory system following rupture of the host hepatocyte. (See life cycle figure.)
 |
|
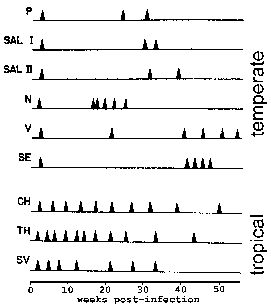
Monkeys were monitored for the timing of relapses following infection with various isolates. P. vivax strains isolated from the Americas (upper) exhibit a "temperate" relapse pattern whereas those from southeast Asia (lower) exhibit a "tropical" relapse pattern. Modified from Contacos et al in AJTMH 21:707, 1972. |
In P. vivax and P. ovale some of the sporozoites do not immediately undergo asexual replication, but enter a dormant phase known as the hypnozoite. (However, some questions about whether P. ovale exhibits the hypnozoite stage have been raised. See Richter et al, 2010, Parasitology Research 107:1285.) This hypnozoite can reactivate and undergo schizogony at a later time resulting in a relapse. Relapse has a specific meaning in regards to malaria and refers to the reactivation of the infection via hypnozoites. Recrudescence is used to describe the situation in which parasitemia falls below detectable levels and then later increases to a patent parasitemia. Interestingly, strains isolated from temperate regions tend to exhibit a longer latent period between the primary infection and the first relapse than strains from tropical regions with continuous transmission (Figure).
Blood Stage. Merozoites released from the infected liver cells invade erythrocytes. The merozoites recognize specific proteins on the surface of the erythrocyte and actively invade the cell in a manner similar to other apicomplexan parasites. (See details on the mechanism of host cell invasion.) After entering the erythrocyte the parasite undergoes a trophic period followed by an asexual replication. The young trophozoite is often called a ring form due to its morphology in Geimsa-stained blood smears. As the parasite increases in size this 'ring' morphology disappears and it is called a trophozoite. During the trophic period the parasite ingests the host cell cytoplasm and breaks down the hemoglobin into amino acids. A by-product of the hemoglobin digestion is the malaria pigment, or hemozoin. (See notes on the food vacuole of Plasmodium.) These golden-brown to black granules have been long recognized as a distinctive feature of blood-stage parasites.
Nuclear division marks the end of the trophozoite stage and the beginning of the schizont stage. Erythrocytic schizogongy consists of 3-5 rounds (depending on species) of nuclear replication followed by a budding process. Late stage schizonts in which the individual merozoites become discernable are called segmenters. The host erythrocyte ruptures and releases the merozoites. These merozoites invade new erythrocytes and initiate another round of schizogony. The blood-stage parasites within a host usually undergo a synchronous schizogony. The simultaneous rupture of the infected erythrocytes and the concomitant release of antigens and waste products accounts for the intermittent fever paroxysms associated with malaria. Blood stage schizogony in P. falciparum differs from the other human malarial parasites in that trophozoite- and schizont-infected erythrocytes adhere to capillary endothelial cells and are not found in the peripheral circulation. This sequestration is associated with cerebral malaria. (See life cycle figure.)
Sexual Stage. As an alternative to schizogony some of the parasites will undergo a sexual cycle and terminally differentiate into either micro- or macrogametocytes. The factors involved in the induction of gametocytogenesis are not known. However, commitment to the sexual stage occurs during the asexual erythrocytic cycle that immediately precedes gametocyte formations. Daughter merozoites from this schizont will develop into either all asexual forms or all sexual forms. Gametocytes do not cause pathology in the human host and will disappear from the circulation if not taken up by a mosquito.
Gametogenesis, or the formation of micro- and macrogametes, is induced when the gametocytes are ingested by a mosquito. After ingestion by the mosquito, the microgametocyte undergoes three rounds of nuclear replication. These eight nuclei then become associated with flagella that emerge from the body of the microgametocyte. This process is readily observable by light microscopy due to the thrashing flagella and is called exflagellation. The macrogametocytes mature into macrogametes. However, at the morphological level this is much less dramatic than the exflagellation exhibited by the microgametocytes.
| Gametogenesis/Exflagellation |
|---|
|
Exflagellation occurs spontaneously when infected blood is exposed to air. Critical factors involved in the induction of this gametogenesis are a decrease in temperature, a decrease in the dissolved carbon dioxide and the subsequent increase in pH to above 8.0 (Box). This somewhat mimics the environmental changes experienced by the gametocytes in that there will be a change to ambient temperature and the gut of the mosquito exhibits a pH of approximately 7.8 as compared to a pH of 7.4 for blood. In addition, a mosquito-derived exflagellation factor (MEF) has also been described and identified as xanthurenic acid, a metabolite from insects. Xanthurenic acid lowers the permissive pH for exflagellation to below 8.0 and is possibly a biological cue for the parasite to undergo gametogenesis (Billker et al, Nature 392:289, 1998; Billker et al, Cell 117:503, 2004).
The highly mobile microgametes will seek out and fuse with a macrogamete. Within 12-24 hours the resulting zygote develops into an ookinete. The ookinete is a motile invasive stage which will transverse both the peritrophic matrix and the midgut epithelium of the mosquito. Transversing the midgut epithelium involves invading and exiting several epithelial cells before emerging on the basal side of the epithelium. The invasion process is similar to other apicomplexa except that the ookinete does not have rhoptries and does not form a parasitophorous vacuole after invading the host cell. (See details on the mechanism of host cell invasion.)
Sporogony. After reaching the extracellular space between the epithelial cells and the basal lamina, the ookinete develops into an oocyst. The oocysts undergo an asexual replication, called sporogony, which culminates in the production of several thousand sporozoites. This generally takes 10-28 days depending on species and temperature. Upon maturation the oocyst ruptures and releases the sporozoites which cross the basal lamina into the hemocoel (body cavity) of the mosquito. (See life cycle figure.)
These sporozoites are motile and have an ability to specifically recognize the salivary glands. After finding the salivary glands the sporozoites will invade and transverse the salivary gland epithelial cells and come to lie within its lumen. Some of these sporozoites will be expelled into the vertebrate host as the mosquito takes a blood meal, and thus reinitiate the infection in the vertebrate host. Although the hemocoel and salivary gland sporozoites are morphologically similar, they are functionally distinct. Salivary gland sporozoites efficiently invade liver cells, but cannot re-invade the salivary glands, whereas the hemocoel sporozoites are inefficient at invading liver cells.
In summary, the malaria parasite exhibits a life cycle with typical apicomplexan features. There are three distinct invasive stages: sporozoite, merozoite and ookinete. All are characterized by apical organelles and can invade or pass through host cells. Two distinct types of merogony are observed. The first, called exoerythrocytic schizogony, occurs in the liver and is initiated by the sporozoite. The resulting merozoites then invade erythrocytes and undergo repeated rounds of merogony called erythrocytic schizogony. Some of the merozoites produced from the erythrocytic schizogony will undergo gamogony. Plasmodium gamogony is described in two phases: gametocytogenesis occuring in the bloodstream of the vertebrate host, and gametogenesis taking place in the mosquito gut. The gametes fuse to become a zygote which first develops into an ookinete and then becomes an oocyst where sporogony takes place.
(For review of life cycle within mosquito see LA Baton, LC Ranford-Cartwright (2005) Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends in Parasitology 21, 573-580.
 |
 |
 |
 |
The pathology and clinical manifestations associated with malaria are almost exclusively due to the asexual erythrocytic stage parasites. Tissue schizonts and gametocytes cause little, if any, pathology. Plasmodium infection causes an acute febrile illness which is most notable for its periodic fever paroxysms occuring at either 48 or 72 hour intervals. The severity of the attack depends on the Plasmodium species as well as other circumstances such as the state of immunity and the general health and nutritional status of the infected individual. Malaria is a chronic disease which has a tendency to relapse or recrudesce (see explanation of difference) over months or even years.
The most common way to obtain malaria is through the natural transmission by mosquitoes (see life cycle). Malaria can also be transmitted via blood transfusions or sharing syringes. Mechanical transmission of infected blood will result in a shorter incubation period since there will be no liver stage. There is also an increased risk of fatality with mechanically-transmitted P. falciparum. The lack of the liver stage infection also precludes relapses in P. vivax or P. ovale infections. Congenital transmission has also been documented, but is believed to be relatively rare despite the heavy infection of the placenta.
| Exoerythrocytic schizogony and prepatent and incubation periods | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Symptoms of malaria usually start to appear 10-15 days after the bite of an infected mosquito. The typical prepatent and incubation periods following sporozoite inoculation vary according to species (Table). The prepatent period is defined as the time between sporozoite inoculation and the appearance of parasites in the blood and represents the duration of the liver stage and the number of merozoites produced. Incubation periods tend to be a little longer and are defined as the time between sporozoite inoculation and the onset of symptoms. Sometimes the incubation periods can be prolonged for several months in P. vivax, P. ovale, and P. malariae. All four species can exhibit non-specific prodromal symptoms a few days before the first febril attack. These prodromal symptoms are generally described as 'flu-like' and include: headache, slight fever, muscle pain, anorexia, nausea and lassitude. The symptoms tend to correlate with increasing numbers of parasites.
These prodromal symptoms will be followed by febrile attacks also known as the malarial paroxysms. These paroxysms will exhibit periodicities of 48 hours for P. vivax, P. ovale, and P. falciparum, and a 72-hour periodicity for P. malariae. Initially the periodicity of these paroxyms may be irregular as the broods of merozoites from different exoerythrocytic schizonts synchronize. This is especially true in P. falciparum which may not exhibit distinct paroxysms, but exhibit a continuous fever, daily attacks or irregular attacks (eg., 36-48 hour periodicity). Patients may also exhibit splenomegaly, hepatomegaly (slight jaundice), and hemolytic anemia during the period in which the malaria paroxysms occur.
| The Malarial Paroxysm | ||||||
|---|---|---|---|---|---|---|
|
The malarial paroxysm (see Table) will usually last 4-8 hours and begins with a sudden onset of chills in which the patient experiences an intense feeling of cold despite having an elevated temperature. This is often referred to as the cold stage and is characterized by a vigorous shivering. Immediately following this cold stage is the hot stage. The patient feels an intense heat accompanied by severe headache. Fatigue, dizziness, anorexia, myalgia, and nausea will often be associated with the hot stage. Next a period of profuse sweating will ensue and the fever will start to decline. The patient is exhausted and weak and will usually fall asleep. Upon awakening the patient usually feels well, other than being tired, and does not exhibit symptoms until the onset of the next paroxysm.
|
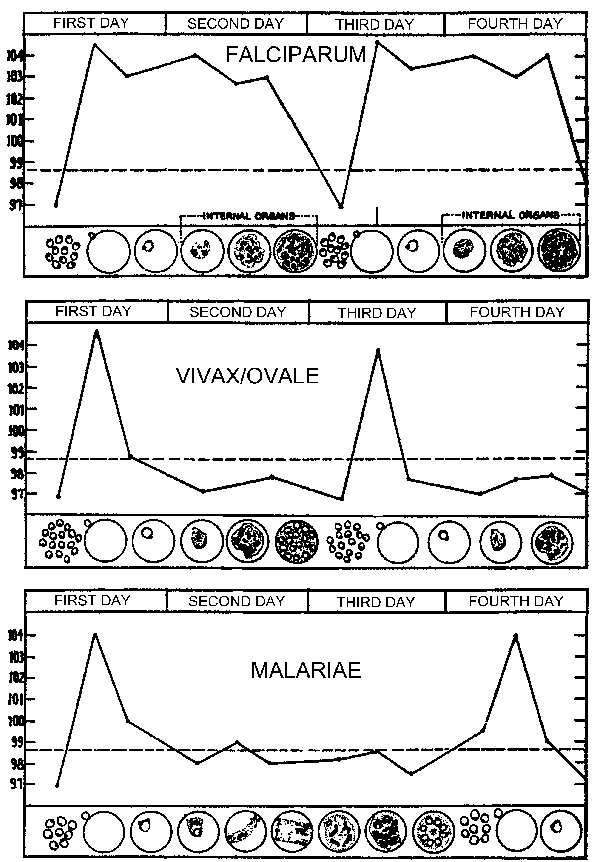
| A typical pattern of temperature (fever) in relation to blood-stage schizogony for the human malarial parasites. The fever paroxysm corresponds to the period of infected erythrocyte rupture and merozoite invasion. (Figure modified from Neva and Brown, Basic Clinical Parasitology, 6th ed., 1994.) |
|
|
|
Body temperature (circles) and TNF levels (triangles) were measured during the malarial paroxysm. The black box denotes the period of intense shivering and the open box denotes profuse sweating. Modified from Karunaweera et al (1992) Proc. Natl. Acad. Sci. 89:3200. |
The periodicity of these paroxysms is due to the synchronous development of the malarial parasite within the human host. In other words, all of the parasites within a host are at approximately the same stage (ie, ring, trophozoite, schizont) as they proceed through schizogony. The malarial paroxysm corresponds to the rupture of the infected erythrocytes and the release of merozoites (Figure above). The 72 hour periodicity in P. malariae is due to its slower growth and maturation during blood-stage schizogony. Studies in P. vivax have demonstrated a correlation between fever and serum TNF-α (tumor necrosis factor-alpha) levels (Figure right). Presumably antigens or toxins are released when the infected erythrocyte ruptures and lead to the production of TNF-α and the febrile attacks.
The severity of the paroxysms and duration of the symptoms varies according to species (see Table below). In general, the severity of the disease correlates with the average and maximum parasitemia exhibited by the various species. P. falciparum is capable of producing a severe and lethal infection, whereas the other species are rarely mortal. Patients infected with P. vivax, especially for the first time, can be quite ill. However, P. vivax rarely causes complications or results in death. On occassion severe malaria involving multiple organs has also been noted in P. vivax infections (see Kochar et al, EID 11 (1), January 2005). Relapses to the activation of P. vivax hypnozoites can occur for several years. P. ovale is the most benign in that the paroxysms tend to be mild and of short duration and relapses seldom occur more than one year after the initial infection. P. malariae generally produces a mild disease, but the initial paroxysms can be moderate to severe. It is the most chronic, though, and recrudescences have been documented several decades after the initial infection. This chronicity is sometimes associated with renal complications, which are probably due to the deposition of antigen-antibody complexes in the glomeruli of the kidney. The malarial paroxysms will become less severe and irregular in periodicity as the host develops immunity. This immunity, however, is not a sterilizing immunity in that the infection persists longer than the symptoms and individuals can exhibit relapses or recrudescences or become reinfected. If untreated, all forms of malaria tend to be chronic.
| Disease Severity and Duration | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||
| Increased morbidity and mortality of falciparum malaria |
|---|
|
In contrast to the other three species, P. falciparum can produce serious disease with mortal consequences. This increased morbidity and mortality is due in part to the high parasitemias associated with P. falciparum infections. These potentially high parasitemias are due in part to the large number of merozoites produced and the ability of P. falciparum to invade all erythrocytes. In contrast, P. vivax and P. ovale prefer reticulocytes (i.e., immature erythrocytes), whereas P. malariae prefers senescent erythrocytes (see species differences). The parasitemia can also rapidly increase due to the cytoadherence and sequestration of P. falciparum. This sequestration in the tissues minimizes removal of infected erythrocytes by the spleen and allows for a more efficient erythrocyte invasion.. The high parasitemia and sequestration result in other complications associated with falciparum malaria, the most notable being anemia and cerebral malaria (discussed in next section). The anemia is due in part to the destruction of erythrocytes during blood-stage schizogony. Furthermore, non-infected erythrocytes are destroyed at higher rates during the infection and there is a decreased production of erythrocytes.
 |
 |
 |
 |
Pathology associated with all malarial species is related to the rupture of infected erythrocytes and the release of parasite material and metabolites, hemozoin (ie, malaria pigment) and cellular debris. In addition to the paroxysms discussed above, the deposition of hemozoin has long been known as a characteristic feature of malaria. There is an increased activity of the reticuloendothelial system, particularly in the liver and spleen and thus their enlargement, as evidenced by macrophages with ingested infected and normal erythrocytes and hemozoin. Except for P. falciparum, the pathology associated with malaria tends to be benign. Several severe complications can be associated with falciparum malaria with cerebral malaria being the most notable and a frequent cause of death.
| Cerebral Malaria |
|---|
|
Cerebral malaria is characterized by an impaired consciousness (Box). The presenting symptoms are severe headache followed by drowsiness, confusion, and ultimately coma. Convulsions are also frequently associated with cerebral malaria. These neurological manifestations are believed to be due to the sequestration of the infected erythrocytes in the cerebral microvasculature. Sequestration refers to the cytoadherence of trophozoite- and schizont-infected erythrocytes to endothelial cells of deep vascular beds in vital organs, especially brain, lung, gut, heart and placenta. This sequestration provides several advantages for the parasite. The major advantage is the avoidance of the spleen and the subsequent elimination of infected erythrocytes. In addition, the low oxygen tensions in the deep tissues may provide a better metabolic environment.
Cytoadherence appears to be mediated by the electron-dense protuberances on the surface of the infected erythrocyte. These 'knobs' are expressed during the trophozoite and schizont stages and are formed as a result of parasite proteins exported to the erythrocyte membrane. Among human Plasmodium species, knobs are restricted to P. falciparum and thus suggest that the knobs play a role in cytoadherence. In addition, there is also a good correlation between animal Plasmodium species which express knobs and exhibit sequestration. Electron microscopy also shows that the knobs are contact points between the infected erythrocyte and the endothelial cell.
|
The molecular mechanisms of cytoadherence involve receptor-ligand interactions. In other words, proteins expressed on the surface of the infected erythrocyte (ligand) will bind to proteins expressed on the surface of the endothelial cells (receptor). PfEMP-1 (erythrocyte membrane protein) is a parasite protein which has been implicated as the cytoadherence ligand (Box). In constrast to the usually highly conserved nature of receptor-ligand interactions, PfEMP-1 is a member of a highly variable (= var) gene family with 40-50 different genes. Several host proteins which possibly function as receptors have been identified (see box below). Many of these host proteins function in cell-cell interactions and are involved in cellular adhesion. Several studies have indicated that the expression of different PfEMP-1 genes is correlated with different receptor-binding phenotypes. This antigenic variation associated with the surface exposed PfEMP-1 allows the parasite to evade the immune system. However, the cytoadherence function is preserved through its ability to recognize multiple receptors (Figure). This antigenic variation may also account for different disease outcomes. For example, intercellular adhesion molecule-1 (ICAM-1) is usually implicated in cerebral pathology.
|
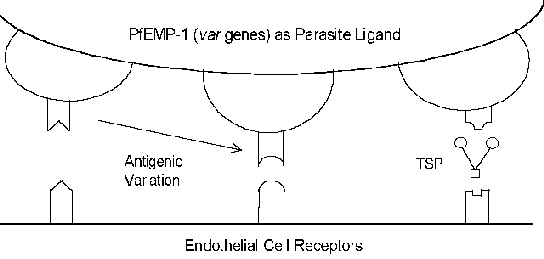
Click here for more on knobs, receptors/ligands, and antigenic variation. |
Early observations of the pathology of cerebral malaria suggested a relationship between large numbers of infected erythrocytes in the microvasculature and the development of the syndrome. Initially it was assumed that the cytoadherence would lead to a mechanical blockage (i.e., cerebral ischemia) and subsequently hypoxia. In addition, the parasite could also cause localized metabolic effects such as hypoglycemia and/or lactic acidosis. The hypoxia and metabolic effects would then cause the coma and subsequent death. However, there are some problems with the sequestration hypothesis:
Neurological sequelae among survivors of cerebral malaria:
|
Because of these problems others have suggested that the coma is mediated by short-lived molecules that affect cerebral function. Possible host mediators include cytokines, such as TNF-a, or nitric oxide. In this cytokine theory, malarial antigens would stimulate TNF-a which could then induce nitric oxide or have other pathological effects. Nitric oxide is known to affect neuronal function and it could also lead to intracranial hypertension through its vasodilator activity. It is unlikely, though, that the systemic release of cytokines would cause coma and one needs to also postulate that release of these mediators in the brain would lead to high local concentrations. In addition, there is minimal lymphocyte infiltration or inflammation associated with the blocked capillaries.
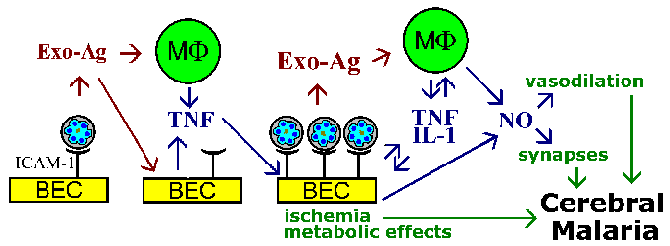
The sequestration hypothesis and cytokine theory for the pathophysiology of cerebral malaria are not mutually exclusive, and both phenomenon are likely to be involved. For example, parasite exo-antigens, which are released at erythrocyte rupture, are known to stimulate macrophages to secrete TNF-a. TNF-a is known to upregulate the expression of adhesion molecules such as ICAM-1 on the surface of brain endothelial cells. This would lead to increase binding of infected erythrocytes and amplify the effects whether they are due to vascular blockage, soluble mediators, metabolic effects, or a combination (Figure).
 |
|
A schematic model depicting some possible mediators of cerebral malaria. The cytoadherence of infected erythrocytes to brain endothelial cells (BEC) and the release of exo-antigens could stimulate the BEC and immune effector cells such a macrophages (MF) to secrete cytokines. These cytokines, such as tumor necrosis factor-a (TNF), would lead to an increased expression of possible endothelial cell receptors (eg., ICAM-1) and promote an increase cytoadherence of infected erythrocytes. Large numbers of bound infected erythrocytes could lead to vascular blockage and hypoxia and have localized metabolic effects (eg., hypoglycemia, lactic acidosis). The increased number of infected erythrocytes and exo-antigens would also lead to higher cytokine levels. TNF-a is also known to stimulate nitric oxide (NO). Nitric oxide can affect neuronal function by interfering with neurotransmission. Nitric oxide also causes vasodilation which could lead to the intracranial hypertension associated with cerebral malaria. Figure adapted from Pasloske and Howard (Annu. Rev. Med. 45:283, 1994). |
In summary:
Reviews on severe malaria and pathogenesis:
 |
 |
 |
 |
| Go to section on: |
Malaria is primarily a disease of the tropics and subtropics and is widespread in hot humid regions of Africa, Asia and South and Central America. The disease was also common in many temperate areas including the USA, Europe and northern Eurasia and Asia, but has been eradicated. In many areas which previously had malaria under control are experiencing a resurgence (see article in The Atlantic). The four human malarial species exhibit an overlapping geographical distribution (Table). P. vivax and P. falciparum are the most commonly encountered species with P. vivax being the most widespread geographically. Mixed infections are common in endemic areas. Molecular methods suggest that P. malariae and P. ovale might be more widespread and prevalent that previously thought (see Mueller et al, Tr. Parasitol. 23:278, 2007).
Geographical Distributions |
||||||||
|---|---|---|---|---|---|---|---|---|
|
| Malaria Epidemiology |
|---|
stable or endemic malaria
|
The epidemiology of malaria can be viewed in terms of being stable (or endemic) or unstable (or epidemic). Stable malaria refers to a situation in which there is a measurable incidence of natural transmission over several years. This would also include areas which experience seasonal transmission. Different areas can experience different levels of incidence rates and this is often denoted by: hypoendemic, mesoendemic, hyperendemic, and holoendemic. Persons living in highly endemic areas usually exhibit a high level of immunity and tolerate the infection well.
Unstable, or epidemic, malaria refers to an increase in malaria in areas of low endemicity or to outbreaks in areas previously without malaria or among non-immune persons. These outbreaks can usually be attributed to changes in human behavior or effects on the environment. For example, human migration and resettlement can either introduce malaria into an area or expose a previously non-immune population to endemic transmission. Changes in the ecology caused by natural disasters or public works projects such as building roads can also impact malaria transmission and lead to epidemics.
| "Everything about malaria is so moulded by local conditions that it becomes a thousand epidemiological puzzles."
Hackett (1937) |
The above quote emphasizes the complexity of malaria and the many facets the disease exhibits. Different communities will experience a different malaria and consequently different control and treatment strategies may be necessary. The intricate interactions between host, parasite, and vector are the major factors in this epidemiological complexity.
For example, as with all vector transmitted diseases, the parasite must be able to establish a chronic infection within the host to maximize the opportunities for transmission. This is especially true in the case of seasonal transmission and in areas of low endemicity. And in general malaria infections are characterized by an initial acute phase followed by a longer relatively asymptomatic chronic phase. This is due in part to the ability of the parasite to avoid complete clearance by the immune system. For example, P. falciparum exhibits an antigenic variation that allows it to stay one step ahead of the immune system. In addition, P. vivax and P. ovale exhibit the hypnozoite stage and are capable of relapses. This allows the parasite to maintain the infection within the human host even after the blood stage of the infection has been cleared. The relative long interval between relapses in some P. vivax isolates probably explains its ability to maintain transmission cycles in some temperate climates.
Several molecular epidemiology studies have indicated that P. falciparum can also produce long-term chronic infections (see Roper et al below).
Roper et al (1996) AJTMH 54:325
|
|
||||||||||||||||||||||||
In regards to the host, humans are the only significant reservoir for the parasite and sustained transmission depends upon maintaining a pool of infected individuals and contact between humans and anopheline mosquitoes. Several factors influence the susceptibility of humans to infection. Obviously the immune status of the individual and their prior experience with malaria will influence the course of the infection. Pregnant women, especially during the first pregnancy, are more susceptible to falciparum malaria as illustrated by a higher prevalence of infection and higher parasitemias. In addition, certain genetic diseases and polymorphisms have been associated with decrease infection or disease (see Innate Resistance).
The potential of the mosquito to serve as a vector depends on the ability to support sporogony, mosquito abundance, and contact with humans, which are all influenced by climatic and ecological factors (Table). The ability to support sporogony is largely dependent upon species in that not all species of Anopheles are susceptible to Plasmodium infection. Temperature and mosquito longevity are other key factors affecting the parasite’s interaction with the vector. Development of P. falciparum requires a minimum temperature of 20oC, whereas the minimum temperature for the other species is 16oC. Temperature also affects the time of development in that the duration of sporogony is substantially shorter at higher temperatures. A shorter duration of sporogony increases the chances that the mosquito will transmit the infection within its lifespan.
Factors Influencing Vectorial Capacity |
||||||
|---|---|---|---|---|---|---|
|
Mosquito density and feeding habits also influence the transmission of malaria. Mosquito density is affected by temperature, altitude, rainfall and the availability of breeding places, whereas human-mosquito contact will be influenced by the mosquito behavior. For example, the degree to which a particular mosquito species is anthropophilic will influence the probability of the mosquito becoming infected and then transmitting the infection to another human. These anthropophilic tendencies are necessarily absolute in that many zoophilic mosquitoes will switch to humans if densities reach high levels or the preferred animal source is diminished. The preferred feeding time and whether the mosquito feeds predominantly indoors or outdoors will influence the transmission dynamics. For example, outdoor feeding mosquitoes are more likely to find a human blood meal in the early evening than those feeding late at night when most people are inside. The behavior of the mosquito also needs to be considered in control activities.
| "Paradoxically, the risks of severe disease in childhood were lowest among populations with the highest transmission intensities, and the highest disease risks were observed among populations exposed to low-to-moderate intensities of transmission."
Snow et al (1997) Lancet 349:1650 |
Persons living in endemic areas do develop immunity against malaria. Almost always a person will exhibit symptoms during their initial exposures to malaria. Symptoms associated with subsequent exposures to malaria are usually less severe, though. The immunity against malaria is slow to develop and requires multiple exposures. In highly endemic areas only young children are at a high risk of developing severe falciparum malaria whereas older children and adults are essentially protected from severe disease and death. However, this immunity is not a sterilizing immunity in that persons can still become infected. In addition the immunity is short lived and in the absence of repeated exposure the level of immunity decreases. For example, previously semi-immune adults will often develop severe malaria upon returning to an endemic area after being in a non-endemic area for 1-2 years. This state of partial immunity in which parasitemia is lowered, but not eliminated, and parasitemia is better tolerated (Figure) is sometimes referred to as premunition. Premunition refers to an immunity that is contingent upon the pathogen being present.
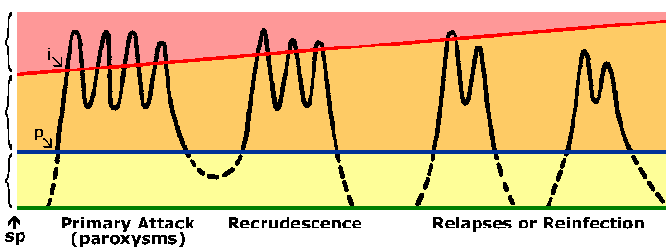 |
|
Diagram representing the course of malaria infection. The black line depicts the blood-stage parasitemia following sporozoite infection (sp). There is prepatent period (p) between sporozoite inoculation and the detection of parasites in the blood. The blue line depicts the microscopic threshold (ie, limit of detection) and the yellow area represents a subpatent parasitemia. The orange area represents an asymptomatic patent parasitemia. The red line depicts a clinical threshold, or the parasitemia which produces paroxysms or other clinical symptoms (pink area). As immunity develops this clinical threshold increases. The incubation period (i) is the time between infection and the appearance of symptoms. |
The immune response could be directed at either the pre-erythrocytic or erythrocytic stages of the parasite’s life cycle. However, the erythrocytic stage of the life cycle is probably the most important in terms of clearing the parasite and lessening the disease. Due to the lack of HLA molecules on the surface of the parasite or the erythrocyte it is usually assumed that antibody will play a key role in blood-stage immunity. Possible effecter mechanisms for antibody include: blocking erythrocyte invasion by merozoites, antibody-dependent cellular killing mediated by cytophilic antibodies, or increased clearance of infected erythrocytes due to binding of antibodies to parasite antigens exposed on the erythrocyte surface. All of these will result in lower parasitemia. The relative importance of these various mechanisms is not clear and probably immunity probably requires the generation of antibodies against numerous targets. This, along with antigenic variation and polymorphisms in many Plasmodium antigens, could explain the slow development of immunity.
The observation that asymptomatic individuals can exhibit high levels of parasitemia has led to the concept of 'anti-disease immunity'. This would be in addition to the ‘anti-parasite’ immunity discussed above which results in lower parasitemias. Severe malaria and death are correlated with TNF-α and other proinflammatory cytokines. As discussed for the paraxoysms and cerebral malaria, antigens or toxins released by the infected erythrocyte could stimulate the production of proinflammatory cytokines. Antibodies against these exo-antigens could possibly neutralize their toxic effects and thus lead to an anti-disease immunity.
Review on Immunity to Malaria:
J Langhorne, FM Ndungu, AM Sponaas, K Marsh (2008) Immunity to malaria: more questions than answers. Nature Immunology 9, 725 - 732.
Because of the difficulties in controlling malaria by other means there is much interest in developing a vaccine against malaria. Currently there is no available vaccine, but there is a substantial research effort directed at identifying vaccine candidates and testing potential vaccines for safety and efficacy. The complex life cycle and biology of the parasite provide several potential targets (Table 15.9). For example, vaccination against the sporozoite or exoerythrocytic stage could prevent infection. However, the induced immunity would need to be completely effective since the escape of a single sporozoite would lead to a blood-stage infection and disease. Vaccines targeted against merozoites or the infected erythrocyte would lower parasitemia by interfering with merozoite invasion or increasing the elimination of infected erythrocytes. Such a vaccine could potentially alleviate much of the pathogenesis associated with malaria even if it were not complete effective. In addition, infection may serve to boost the immune response. It may be possible to vaccinate against the disease by immunizing against potentially toxic antigens. Antibodies neutralizing antigens that stimulate a proinflammatory immune response may lessen some of the pathogenesis associated with malaria. Sexual stages of the parasite such as gametocytes and gametes could also be targeted. Antibodies directed against gamete antigens can prevent infection of the mosquito and sporogony. Such a vaccine would be altruistic in that it would not protect the individual against disease, but protect others in the community by lowering the transmission.
| Potential Vaccine Strategies | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| Innate Resistance |
|---|
|
Certain genetic diseases and polymorphisms have been associated with decrease infection or disease (Box). For example, individuals which lack the Duffy blood-group antigen are refractory to P. vivax. A large proportion of the populations in western Africa are Duffy negative, thus accounting for the low levels of P. vivax in west Africa. This innate resistance led to the identification of the Duffy antigen as the erythrocyte receptor for merozoite invasion. (See receptor-ligand interactions during merozoite invasion.)
Several inherited erythrocyte disorders are found predominantly in malaria endemic areas and at frequencies much higher than expected. This has lead to speculation that these disorders confer some protection against malaria. For example, southeast Asian ovalcytosis is due to a mutation in an erythrocyte membrane protein called band 3. This mutation causes the erythrocyte membrane to become more rigid and more refractory to merozoite invasion. The mechanism(s) by which the other diseases might confer protection against malaria are not known. In most cases it is presumed or speculated that the combination of the defect and infection leads to premature lysis or clearance of the infected erythrocyte. For example, glucose-6-phosphate dehydrogenase (G6PD) deficient erythrocytes would have an impaired ability to handle oxidative stress (see Drug Action). The additional oxidants produced as a result of parasite metabolism and the digestion of hemoglobin (see Biochemistry Notes) may overwhelm the infected erythrocyte and lead to its destruction before the parasite is able to complete schizogony. Sickle cell anemia and thalassemia are also speculated to make the infected erythrocyte more susceptible to oxidative stress.
Reviews on red blood cell polymorphisms and malaria:
| Factors leading to a decline in malaria transmission in the U.S. |
|---|
|
Malaria was previously more widespread in temperate areas including North America and Europe. It is believed that malaria was introduced to the Americas by the European colonists (P. vivax and P. malariae) and African slaves (P. falciparum) during the 16th and 17th centuries. Malaria became endemic in many parts of the United States excluding deserts and mountainous areas and the incidence probably peaked around 1875. A population shift from rural to urban areas, drainage of swamps to create farmland, improved housing and nutrition, better socioeconomic conditions and standards of living, greater access to medical services, and the availability of quinine for treatment all contributed to the decline in the prevalence of malaria even before the introduction of specific control measures (Box). Some control activities, such as case detection and treatment, larviciding and house spraying, were introduced during the 1940's and led to the eradication of malaria in the United States. Since the 1950's nearly all cases of malaria in the U.S. have been imported. The major factors contributing to this eradication appear to be a population shift from rural to urban areas and an increase in the standard of living, which resulted in improved housing, better nutrition, and greater access to medical services.
The vast majority of malaria cases diagnosed in the United States are acquired by persons while traveling to countries where malaria is endemic. However, during the 1990's there were several outbreaks of autochthonous malaria transmission in the U.S. (Zucker, 1996). These outbreaks were associated with densely populated areas and large numbers of immigrants. More than 80% of the cases were P. vivax. In addition, the outbreaks were associated with unusually hot and humid weather, which may increase anopheline survival and decrease the duration of the sporogonic cycle, thus allowing for the development of infective sporozoites. (See also recent report on seven cases of locally acquired P. vivax malaria that occurred in Florida during July-August 2003, MMWR 52:908.)
reduce human-mosquito contact
|
Strategies for preventing and controlling malaria involve three different approaches (see Box). Prevention of malaria in individuals will generally involve the reduction of human-mosquito contact through the use of bednets, repellents, etc. Chemoprophylaxis (see below) can also be used, especially in travelers. However chemoprophylaxis only suppresses parasitemia and does not prevent infection.
Control activities at the community level can utilize approaches which directly reduce human-mosquito contact as well as approaches which reduce the total number of mosquitoes in an area. Such approaches include the reduction in mosquito breeding grounds (eg, environmental modification), target the larva stages with chemical or biological agents, and massive insecticide spraying for the adult mosquitoes. Biological control methods include the introduction of fish which eat the mosquito larvae or bacteria (eg, Bacillus thuringiensis) which excrete larval toxins. Case detection and treatment is another potential control method. Identifying and treating infected persons, especially asymptomatic individuals, will reduce the size of the parasite reservoir within the human population and can lower transmission rates. However, this can be a relatively expensive approach.
These approaches are not mutually exclusive and can be combined. Many of the successful control programs include both measures to control mosquitoes and treatment of infected individuals. There is no standard method of malaria control that has proven universally effective. The epidemiologic, socioeconomic, cultural and infrastructural factors of a particular region will determine the most appropriate malaria control. Some of the factors which need to be considered include:
The control of malaria in tropical Africa has been particularly problematic because of the high transmission rates and the overall low socio-economic level. Several studies have shown that insecticide treated bednets (ITBN) reduce the morbidity and mortality associated with malaria. In most areas the introduction of bednets do not require large promotional programs and their use is readily accepted. This may be in part due to the reduction in mosquito nuisance biting. Some questions have been raised in regards to the economic sustainability of bednet programs. It is necessary to re-treat the bednets with insecticide periodically and the bednets need to be repaired and replaced as they become torn and wear out. In addition, some have raised concerns about the long-term benefits of bednets since they reduce exposure, but do not eliminate it. This reduction in exposure may delay the acquisition of immunity and simply pospone morbidity and mortality to older age groups.
[Review on malaria control: R.S. Phillips (2001) Current status of malaria and potential for control. Clin. Microbiol. Rev. 14:208.]
|
Malaria is suspected in persons with a history of being in an endemic area and presenting symptoms consistent with malaria (see Clinical Manifestations). These symptoms, especially in the early stages of the infection, are non-specific and often described as flu-like. As the disease progresses, the patient may exhibit an enlarged spleen and/or liver and anemia. Diagnosis is confirmed by microscopy. Thick blood smears are generally more sensitive for the detection of parasites, whereas thin smears are preferable for species identification. (See blood-stage morphology of Plasmodium species.) If parasites are not found on the first blood smear it is recommended to make additional smears every 6-12 hours for as long as 48 hours. A tentative diagnosis of P. falciparum (numerous and exclusively ring stages) could constitute a medical emergency, especially in a non-immune person. Rapid immunochromatographic tests (ie, dipsticks) based on antigen detection are also available (see review).
 |
 |
 |
 |
Several antimalarial drugs are available. Many factors are involved in deciding the best treatment for malaria. These factors include the parasite species, the severity of disease (eg., complicated), the patient's age and immune status, the parasite's susceptibility to the drugs (i.e., drug resistance), and the cost and availability of drugs. Therefore, the exact recommendations will often vary according to geographical region. In addition, the various drugs act differentially on the different life cycle stages (Table). Other links of interest:
| Selected Antimalarial Drugs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Fast-acting blood schizontocides, which act upon the blood stage of the parasite, are used to treat acute infections and to quickly relieve the clinical symptoms. Chloroquine is generally the recommended treatment for patients with P. vivax, P. ovale, P. malariae, and uncomplicated chloroquine-sensitive P. falciparum infections. Chloroquine is safe and usually well tolerated. Side effects may include pruritus (i.e., itching), nausea, or agitation. Patients infected with either P. vivax or P. ovale, and that are not at a high risk for reinfection, should also be treated with primaquine (a tissue schizontocide). Primaquine is effective against the liver stage of the parasite, including hypnozoites (see relapses), and will prevent future relapses. The combination of chloroquine and primaquine is often called 'radical cure'.
Severe, or complicated, falciparum malaria is a serious disease with a high mortality rate and must be regarded as life threatening, and thus requires urgent treatment. Treatment typically requires parenteral drug administration (i.e., injections) since the patients are often comatose or vomiting, and thus cannot take the drugs orally. Parenteral formulations are available for chloroquine, quinine, quinidine and artemisinin derivatives. The artemisinin derivatives are generally the preferred choice, but are not yet approved everywhere. For example, in the United States quinine and quinidine are the approved drugs for severe malaria. Patients need to be continuously monitored for hematocrit, parasitemia, hydration levels, hypoglycemia, and signs of drug toxicity and other complications during the course of treatment. A switch to oral administration should be made as soon as the patient is able. Most deaths due to severe malaria occur at or close to home in situations where the patients cannot be taken to the hospital. Artemisinin suppositories which can be administered by village health workers have also been developed and have proved to be safe and effective.
The efficacy of chloroquine is greatly diminished by the wide spread chloroquine resistance of P. falciparum and the emergence of chloroquine-resistant P. vivax. If chloroquine therapy is not effective, or if in an area with chloroquine-resistant malaria, common alternative treatments include: mefloquine, quinine in combination with doxycycline, or Fansidar®. Derivatives of artemisinin (dihydroartemisinin, artesunate and artemether) are increasingly used in Asia and Africa and are now recommend as the first line of treatment by the World Health Organization. These drugs were originally derived from the wormwood plant (Artemesia annua) and have been used for a long time in China as an herbal tea called quinhaosu to treat febrile illnesses. To prevent the high recrudescence rates associated with artemisinin derivatives and to slow the development of drug resistance it is recommended that treatment be combined with an unrelated anti-malarial. Drugs used in combination with artemisinin include mefloquine, lumefantrine, Fansidar®, and amodiaquine.
Chemoprophylaxis. Chemoprophylaxis is especially important for persons from non-malarious areas who visit areas endemic for malaria. Such non-immune persons can quickly develop a serious and life-threatening disease. As in the case of treatment there is no standard recommendation and the choices for chemoprophylaxis are highly dependent upon the conditions associated with the travel and the indivdual person. (See CDC Factsheet on Preventing Malaria.) Chemoprophylaxis requires the use of non-toxic drugs since these drugs will be taken over extended periods of time. Generally the patient will start to take the drug before traveling and then continue taking the drug during the stay in the endemic area and continue taking the drug after returning. This is to insure the drug is maintained at sufficient levels throughout out the visit and to protect against any infection obtained during the visit. Unfortunately, many of the effective and non-toxic drugs (eg, chloroquine, pyrimethamine, proquanil) are of limited use because of drug resistance. Another strategy is presumptive (or 'standby') treatment to be used in conjunction with prophylaxis. In this case a person either forgoes prophylaxis or takes chloroquine or another relatively non-toxic drug for prophylaxis and carries a drug like Fansidar, mefloquine, or quinine, which they will take if they start to exhibit symptoms associated with malaria.
The use of mefloquine for malaria chemoprophylaxis is somewhat controversial. Mefloquine is efficacious at preventing malaria with a single does per week, thus offering advantages to drugs that need to be administered daily. At this dosage mefloquine is tolerated by most individuals. However, some people experience neuropsychiatric adverse affects such as sleep disturbances and nightmares. This could be exacerbated by international travel which is a stressful event. Randomized, blinded and controled trials indicate that neuropsychiatric adverse affects are only slightly higher with mefloquine than with other anti-malarials.
Killing the exoerythrocytic stage (i.e., liver) would prevent the blood infection and is known as causal prophylaxis. This is highly desirable in that it limits the amount of time the prophylactic drug needs to be taken before and after travel to an endemic area. The only currently available drug for causal prophylaxis is primaquine. However, malaria prophylaxis is not an approved use of primaquine and should only be prescribed for prophylaxis on a case-by-case basis. For example, for persons who frequently have trips of short duration to highly endemic areas and that the person does not exhibit glucose-6-phosphate dehydrogenase deficiency. Tafenoquine is currently undergoing field evaluation for its use in causal prophylaxis.
Reviews on the treatment of malaria:
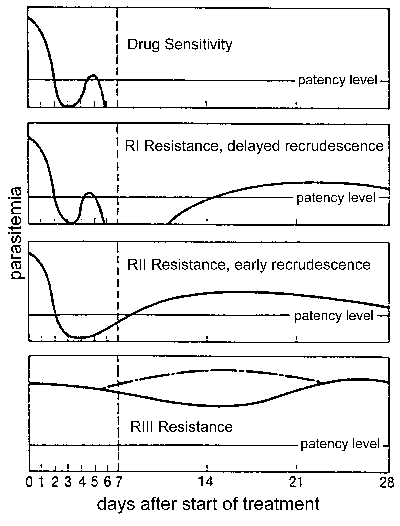
Drug resistance, and in particular, chloroquine resistance is a major public health problem in the control of malaria. Drug resistance is defined by a treatment failure and can be graded into different levels depending on the timing of the recrudescence following treatment (Figure). Traditionally these levels of drug resistance have been defined as sensitive (no recrudescence), RI (delayed recrudescence), RII (early recrudescence), and RIII (minimal or no anti-parasite effect). A modified protocol based on clinical outcome was introduced by WHO in 1996. In this protocol the level of resistance is expressed as adequate clinical response (ACR), late treatment failure (LTF), or early treatment failure (ETF) as defined by the following:
Either protocol can be used to determine drug resistance, but the clinical outcome protocol is more practical in areas of intense transmission where it may be difficult to distinguish re-infection from recrudescence and where parasitemia in the absence of clinical symptoms is common. Drug resistance by either protocol is determined with in vivo tests in which patients are hospitalized and monitored during and following standard drug treatment. There are also in vitro tests that can estimate the level of drug resistance by determining the efficacy of the drugs against P. falciparum grown in culture (see Drug Resistance: Malaria at WHO). The in vivo and in vitro tests do not always correspond since host immunity and other factors can affect the in vivo outcomes. The identification of specific mutations which might be associated with drug resistance (see Table on other page) may also lead to the development of tests based on molecular markers.
Drug resistance develops when parasites with decreased sensitivities to antimalarial drugs are selected under drug pressure. Decreased drug sensitivity can be conferred by several mechanisms (see Mechanisms of Drug Resistance) and reflects genetic mutation(s) or polymorphisms in the parasite population. The drug-resistance parasites will have a selective advantage over the drug-sensitive parasites in the presence of drug and will be preferentially transmitted. Major factors in the development of drug resistance are the use of subtherapeutic doses of drugs or not completing the treatment regimen (Table). The lower drug levels will eliminate the most susceptible parasites, but those which can tolerate the drug will recover and reproduce. Over time this will lead to a continued selection for parasites which can tolerate even higher doses of the drug. It is crucial to maintain an adequate concentration of the drug for a sufficient time to completely eliminate the parasites from any given individual.
| Factors Contributing to Development and Spread of Drug Resistance |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chloroquine resistance. After its introduction near the end of World War II, chloroquine quickly became the drug of choice for the treatment and prevention of malaria. Not only is chloroquine an effective drug--probably due to its site of action in the food vacuole and its interference with hemozoin formation (see drug action)--but it is also relatively non-toxic and cheap. Two foci of chloroquine resistant P. falciparum were detected in Colombia and at the Cambodia-Thailand border during the late 1950's. During the 1960's and 1970's, resistant parasites spread through South America, Southeast Asia, and India. Resistance was first reported in east Africa in 1978 and spread throughout the continent during the 1980's. Chloroquine resistant P. vivax was not reported until 1989 in Papua New Guinea and is now found in several foci in southeast Asia and perhaps South America.
The basis of chloroquine resistance is reduced chloroquine accumulation in the parasite's food vacuole. Furthermore, chloroquine resistance can be partially reversed with inhibitors of P-glycoprotein (an ABC transporter) which are responsible for multi-drug resistance (MDR) in tumor cell lines, thus suggesting a similar phenomenon may occur in Plasmodium. Mutations in a MDR-like gene from P. falciparum (Pfmdr1) were implicated in chloroquine resistance. However, these mutations are not predictive of chloroquine resistance in all geographical areas. PfMDR1 appears to contribute to the degree of chloroquine resistance, but alone it is insufficient to confer resistance. However, PfMDR1 does appear to play a role in resistance to mefloquine and halofantrine and influences the sensitivity to artemisinin.
Another candidate for the genetic locus of chloroquine resistance was identified through a genetic cross and mapping experiment. A 400 kb region on chromosome 7 was found to segregate with chloroquine resistance and further analysis suggested that a single gene, called Pfcrt, was responsible for chloroquine resistance. Out of a total of 10 polymorphisms identified in this gene, only a single mutation is perfectedly associated with the chloroquine resistance phenotype. This mutation results in a lysine at residue 76 being changed to a threonine (K76T). Several field studies have demonstrated an association between Pfcrt-K76T and chloroquine resistance using both in vivo and in vitro methods. It has been recently suggested that there have been at least 4 founder mutations in the Pfcrt gene associated with different geographical regions: Asia/Africa, Papua New Guinea, Brazil/Peru, and Colombia (Wootton et al, Nature 418:320, 2002). Presumably the use of chloroquine resulted in the subsequent selection and spread of the resistant phenotype.
Reviews on drug resistance:
These pages are developed and maintained by Mark F. Wiser, Tulane University (©2000). Last update on September 25, 2020 .