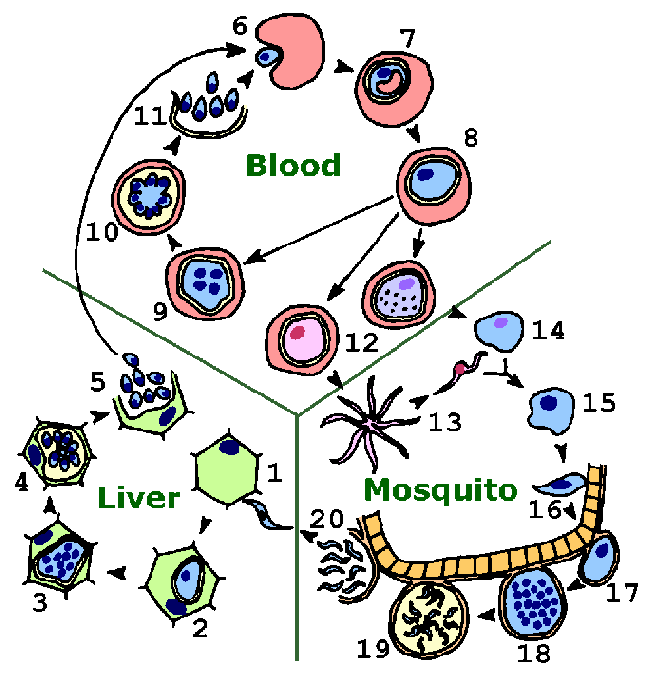
The malaria parasite exhibits a complex life cycle involving an insect vector (mosquito) and a vertebrate host (human). (See also file in pdf format for printing.) The major phases of the life cycle are: liver stage, blood stage, sexual stage, and sporogony.

(Return to Life Cycle Section in main document.)
The infection is initiated when sporozoites are injected with the saliva of a feeding mosquito. Sporozoites are carried by the circulatory system to the liver and invade hepatocytes (1). The intracellular parasite undergoes an asexual replication known as exoerythrocytic schizogony within the hepatocyte (2-4). Exoerythrocytic schizogony culminates in the production of merozoites which are released into the bloodstream (5). A proportion of the liver-stage parasites from P. vivax and P. ovale go through a dormant period (not shown) instead of immediately going through the asexual replication (ie, temporarily stay at step 2). These hypnozoites will reactivate after several weeks to months (or years) after the primary infection and are responsible for relapses. (Return to relapses in main document.)
Merozoites invade erythrocytes (6) and undergo a trophic period in which the parasite enlarges (7-8). The early trophozoite is often referred to as 'ring form' because of its morphology. Trophozoite enlargement is accompanied by an active metabolism including the ingestion of host cytoplasm and the proteolysis of hemoglobin into amino acids. The end of the trophic period is manifested by multiple rounds of nuclear division without cytokinesis resulting is a schizont (9). Merozoites bud from the mature schizont, also called a segmenter (10), and the merozoites are released following rupture of the infected erythrocyte (11). Invasion of erythrocytes reinitiates another round of the blood-stage replicative cycle (6-11). (Return to blood-stage schizogony in main document.)
The intermittent fevers often associated with malaria are due to the synchronous rupture of infected erythrocytes and release of merozoites (see malarial paroxysm). Trophozoite- and schizont-infected erythrocytes are rarely found in the peripheral circulation during P. falciparum infections. Erythrocytes infected with these stages adhere to endothelial cells and sequester in the microvasculature of vital organs, especially brain, heart and lungs. Sequestration in the brain is a contributing factor in cerebral malaria.
As an alternative to the asexual replicative cycle, the parasite can differentiate into sexual forms known as macro- or microgametocytes (12). The gametocytes are large parasites which fill up the erythrocyte, but only contain one nucleus. Ingestion of gametocytes by the mosquito vector induces gametogenesis (i.e., the production of gametes) and escape from the host erythrocyte. Factors which participate in the induction of gametogenesis include: a drop in temperature, an increase in carbon dioxide, and mosquito metabolites. Microgametes, formed by a process known as exflagellation (13), are flagellated forms which will fertilize the macrogamete (14) leading to a zygote (15). (Return to sexual replication in main document.)
The zygote develops into a motile ookinete (16) which penetrates the gut epithelial cells and develops into an oocyst (17). The oocyst undergoes multiple rounds of asexual replication (18) resulting in the production of sporozoites (19). Rupture of the mature oocyst releases the sporozoites into the hemocoel (body cavity) of the mosquito (20). The sporozoites migrate to and invade the salivary glands (not shown), thus completing the life cycle. (Return to sporogony in main document.)
In summary, the malaria parasites undergoes three distinct asexual replicative stages (exoerythrocytic schizogony, blood stage schizogony, and sporogony) resulting in the production of invasive forms (merozoites and sporozoites). A sexual reproduction occurs with the switch from vertebrate to invertebrate host and leads to the invasive ookinete. All invasive stages are characterized by the apical organelles typical of apicomplexan species. The invasive stages differ in regards to the types of cells or tissues they invade and their motility. Following the successful invasion, a few of these invasive forms will undergo a high level of proliferation to establish the infection in the new host. (See Rosenberg, 2008 for a discussion of the numbers.)
 |
|